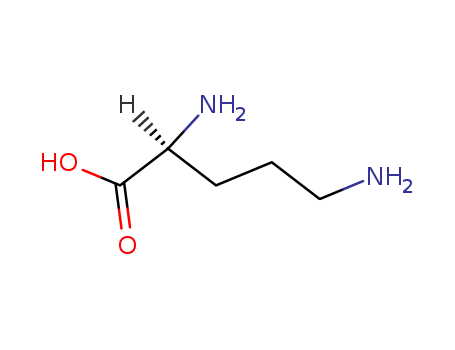
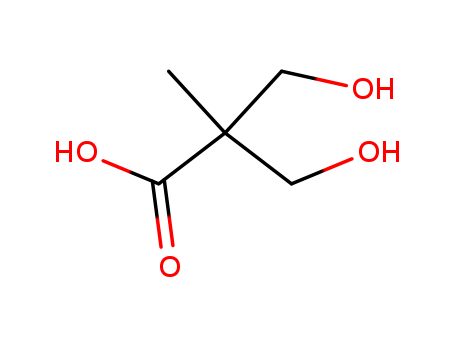
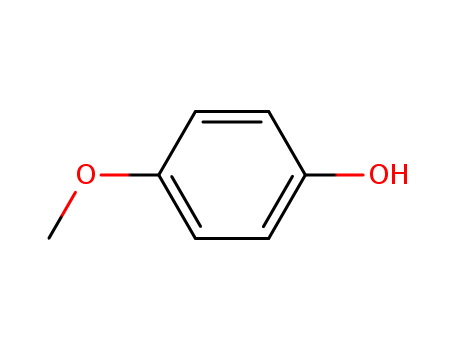
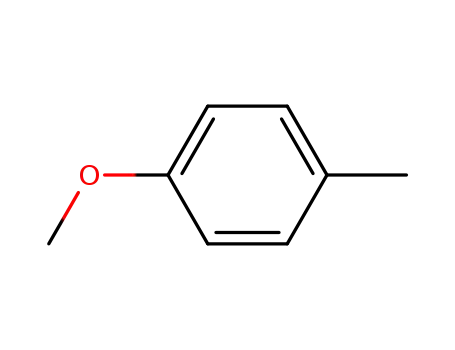
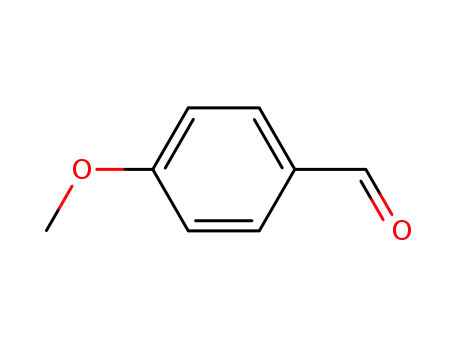
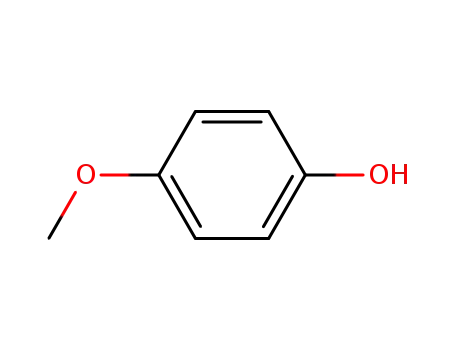
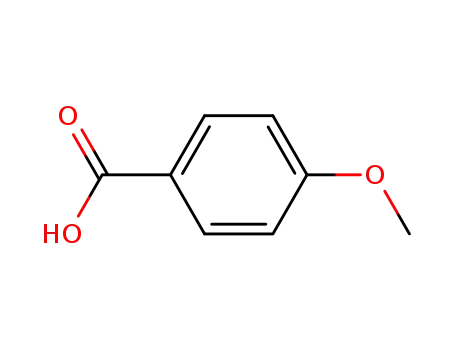
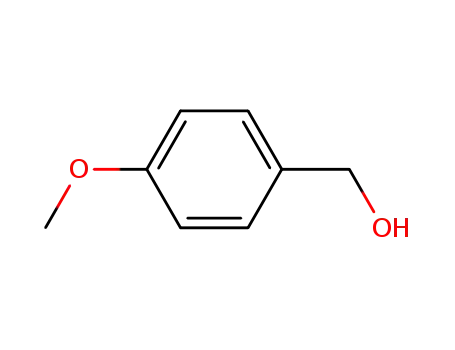
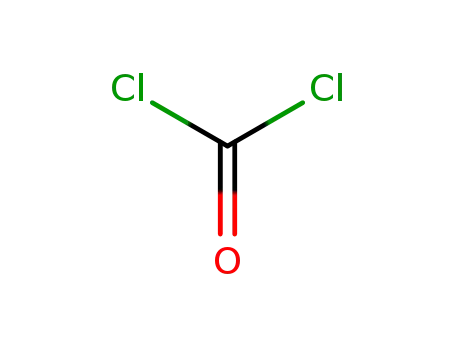
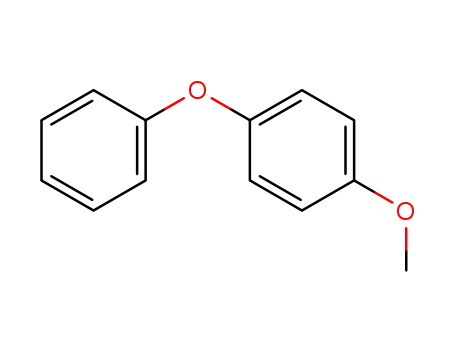
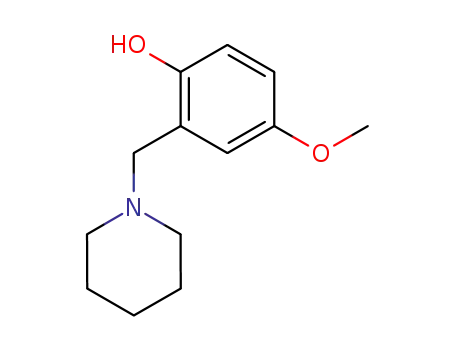
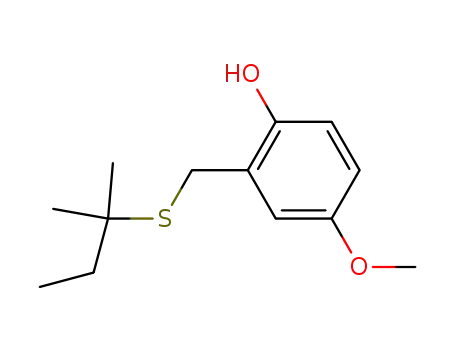
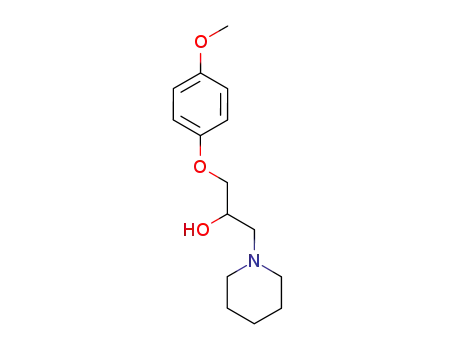
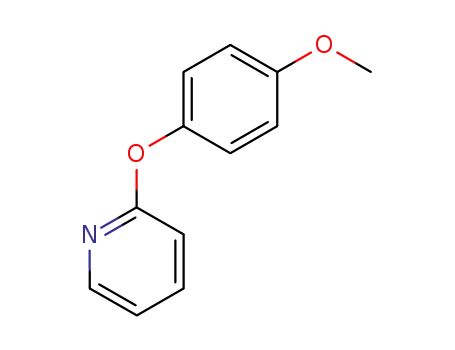
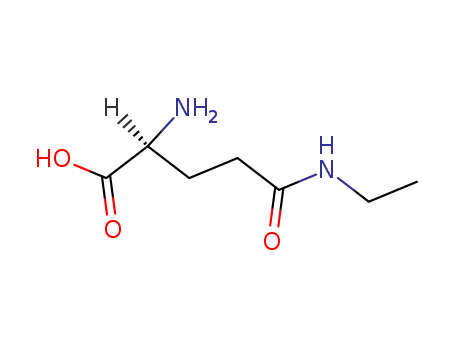
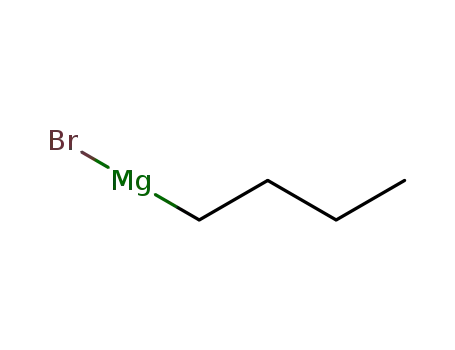Description of Solvent Dependence of Rate Constants in Terms of Pairwise Group Gibbs Function Interaction Parameters. Medium Effects for Hydrolysis of p-Methoxyphenyl Dichloroacetate in Aqueous Solutions Containing Urea and Alkyl-Substituted Ureas
Blokzijl, Wilfried,Engberts, Jan B. F. N.,Jager, Jan,Blandamer, Michael J.
, p. 6022 - 6027 (1987)
Rate constants for neutral hydrolysis of...
Inhibition of Water-Catalyzed Ester Hydrilysis in Hydrophobic Microdomains of Poly(methacrylic acid) Hypercoils
Jager, Jan,Engberts, Jan B. F. N.
, p. 3331 - 3334 (1984)
The water-catalyzed hydrolysis of p-meth...
Kinetics of hydrolysis of 4-methoxyphenyl-2,2-dichloroethanoate in binary water-cosolvent mixtures; the role of solvent activity and solute-solute interactions
Rispens, Theo,Cabaleiro-Lago, Celia,Engberts, Jan B. F. N.
, p. 597 - 602 (2005)
Rate constants are reported for the pH-i...
The mechanism of hydrolysis of aryl ether derivatives of 3-hydroxymethyltriazenes
Carvalho, Emilia,Francisco, Ana Paula,Iley, Jim,Rosa, Eduarda
, p. 2056 - 2063 (2005)
1-Aryl-3-aryloxymethyl-3-methyltriazenes...
Novel photo-fragmentation of 3,3,6,6-tetra(p-methoxyphenyl)-1,2-dioxane through a C-O bond cleaved 1,6-diradical intermediate
Kamata, Masaki,Tanaka, Takehito,Kato, Mitsuaki
, p. 8181 - 8184 (1996)
Photolysis and thermolysis of 3,3,6,6-te...
Gold nanoparticles stabilized by graphene quantum dots as catalysts for C–C bond cleavage in β-O-4 lignin model compounds
Zhang, Fangwei,Zhang, Jiali,Guo, Shouwu
, p. 105 - 109 (2019)
In this work, we report oxidative cleava...
Efficiency of 2.45 and 5.80 GHz microwave irradiation for a hydrolysis reaction by thermostable β-Glucosidase HT1
Nagashima, Izuru,Sugiyama, Jun-Ichi,Sakuta, Tomomi,Sasaki, Masahide,Shimizu, Hiroki
, p. 758 - 760 (2014)
Microwave irradiation at different frequ...
Isobaric and Isochoric Activation Parameters for the Water-Catalyzed Hydrolysis of p-Methoxyphenyl 2,2-Dichloropropionate in Typically Aqueous Solutions
Holterman, Herman A. J.,Engberts, Jan B. F. N.
, p. 6382 - 6384 (1982)
Isobaric thermodynamic activation parame...
Origins of high catalyst loading in copper(i)-catalysed Ullmann-Goldberg C-N coupling reactions
Sherborne, Grant J.,Adomeit, Sven,Menzel, Robert,Rabeah, Jabor,Brückner, Angelika,Fielding, Mark R.,Willans, Charlotte E.,Nguyen, Bao N.
, p. 7203 - 7210 (2017)
A mechanistic investigation of Ullmann-G...
Aryldiazonium Salts as Photo-affinity Labelling Reagents for Proteins
Kieffer, Brigitte L.,Goeldner, Maurice Ph.,Hirth, Christian G.
, p. 398 - 399 (1981)
Aryldiazonium tetrafluoroborates, substi...
REMOVAL OF O-AND N-BENZYL GROUPS BY FUNGAL BIOTRANSFORMATION
Holland, Herbert L.,Conn, Morgan,Chenchaiah, P. Chinna,Brown, Frances M.
, p. 6393 - 6394 (1988)
Biotransformation by resting cultures of...
Microbial Oxidation of Racemic vic-Diols. Synthesis of (R)- and (S)-α-Hydroxypropiophenones
Ohta, Hiromichi,Yamada, Hiroshi,Tsuchihashi, Gen-ichi
, p. 2325 - 2326 (1987)
Both enantiomers of 2-hydroxy-1-phenyl-1...
Synthesis of Tetrahydroazocino- and Dihydroazepino-1,2-Benzoquinones via Amino-Claisen Rearrangement of 4-(2-Vinyl-Azetidino and Aziridino)-1,2-Benzoquinones
Viallon, Loik,Reinaud, Olivia,Capdevielle, Patrice,Maumy, Michel
, p. 4787 - 4790 (1995)
Amino-Claisen rearrangement of 4-(2-viny...
Rate parameter changes by added albumin in the microsomal oxidative demethylation of deuteriated and non-deuteriated 4-methoxyanisole
Masuda, Masatoshi,Kishimoto, Daisuke,Kurihara, Norio
, p. 806 - 810 (1996)
Bovine serum albumin (BSA) added to the ...
Pairwise Gibbs energies of interaction involving N-alkyl-2- pyrrolidinones and related compounds in aqueous solution obtained from kinetic medium effects
Apperloo, Joke J.,Streefland, Lisette,Engberts, Jan B. F. N.,Blandamer, Michael J.
, p. 411 - 418 (2000)
Kinetic solvent effects of N-alkyl-2-pyr...
Stereoselective umpolung tandem addition of heteroatoms to phenol
Todd, Michael A.,Sabat, Michal,Myers, William H.,Smith, Timothy M.,Harman, W. Dean
, p. 6906 - 6907 (2008)
Upon coordination to {TpW(PMe3)(NO)}, ph...
-
Bhatt
, p. 221,222,224 (1978)
-
The mechanisms of hydrolysis of N-alkyl O-arylthioncarbamate esters
Humeres, Eduardo,De Souza, Eduardo P.,Debacher, Nito A.
, p. 915 - 924 (2010)
The hydrolysis of N-ethyl O-p-methoxyphe...
2-PHENOXYCHROMONES AND A STRUCTURALLY RELATED FLAVONE FROM LEAVES OF ROSA RUGOSA
Hashidoko, Yasuyuki,Tahara, Satoshi,Mizutani, Junya
, p. 3837 - 3838 (1991)
Key Word Index - Rosa rugosa; Rosaceae; ...
Photohomolysis and Photoheterolysis in Aryl Sulfonates and Aryl Phosphates
Bonesi, Sergio,Protti, Stefano,Fagnoni, Maurizio
, p. 6315 - 6323 (2021)
The photochemical behaviour of selected ...
Hydrolysis of Two Acyl Activated Esters in Water-Rich 2-n-Butoxyethanol-Water Mixtures. Effects of Hydrophobic Interactions on Enthalpies, Entropies, and Heat Capacities of Activation
Holterman, Herman A. J.,Engberts, Jan B. F. N.
, p. 4256 - 4257 (1980)
-
Are phosphines viable ligands for Pd-Catalyzed aerobic oxidation reactions? Contrasting insights from a survey of six reactions
Tereniak, Stephen J.,Landis, Clark R.,Stahl, Shannon S.
, p. 3708 - 3714 (2018)
Phosphines are the broadest and most imp...
Pronounced axial thiolate ligand effect on the reactivity of high-valent oxo-iron porphyrin intermediate
Urano,Higuchi,Hirobe,Nagano
, p. 12008 - 12009 (1997)
-
Stereodivergent Synthesis of β-Heteroatom-Substituted Vinyl-silanes by Sequential Silylzincation-Copper(I)-Mediated Electrophilic- Substitution
Fopp, Carolin,Isaac, Kevin,Romain, Elise,Chemla, Fabrice,Ferreira, Franck,Jackowski, Olivier,Oestreich, Martin,Perez-Luna, Alejandro
, p. 724 - 735 (2017)
Sulfur-, oxygen-, and phosphorus-substit...
Direct conversion of aryl halides to phenols using high-temperature or near-critical water and microwave heating
Kormos, Chad M.,Leadbeater, Nicholas E.
, p. 4728 - 4732 (2006)
The direct conversion of aryl halides to...
Engineering a highly improved porous photocatalyst based on Cu2O by a synergistic effect of cation doping of Zn and carbon layer coating
Yuan, Yusheng,Sun, Li-Ming,Gao, Hao,Mo, Sha,Xu, Tianyi,Yang, Lei,Zhan, Wen-Wen
, p. 16010 - 16015 (2020)
Zn-doped cuprous oxide (Cu2O) nanopartic...
Coordination Polymers as a Functional Material for the Selective Molecular Recognition of Nitroaromatics and ipso-Hydroxylation of Arylboronic Acids
Rani, Pooja,Husain, Ahmad,Bhasin,Kumar, Girijesh
, (2021/12/06)
We report the synthesis and structural c...
A Nanographene-Based Two-Dimensional Covalent Organic Framework as a Stable and Efficient Photocatalyst
Addicoat, Matthew A.,Bonn, Mischa,Chen, Qiang,Fu, Shuai,Graf, Robert,Hanayama, Hiroki,Jin, Enquan,Landfester, Katharina,Müllen, Klaus,Narita, Akimitsu,Wang, Hai I.,Wei, Wenxin,Zhang, Kai A. I.
supporting information, (2021/12/22)
Synthesis of covalent organic frameworks...
Imidazolium-urea low transition temperature mixtures for the UHP-promoted oxidation of boron compounds
Martos, Mario,Pastor, Isidro M.
, (2022/01/03)
Different carboxy-functionalized imidazo...
Reusable and Efficient Polystyrene Immobilized Ionic Liquid Catalyst for Batch and Flow Methylation of Hydroquinone
Bhongale, Priyanka V.,Joshi, Sunil S.,Mali, Nilesh A.
, (2022/01/31)
An environmentally benign process for sy...
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego