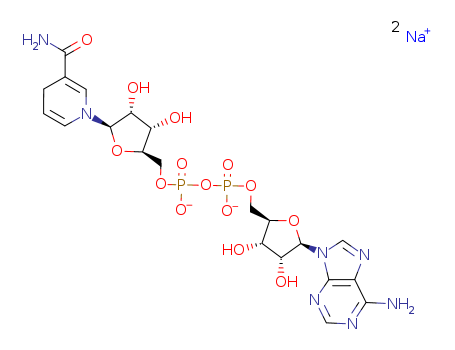
Hot Sale Trustworthy Factory Supply Nicotinamide Adenine Dinucleotide 606-68-8 with Low Price
- Molecular Formula:C21H27N7Na2O14P2
- Molecular Weight:709.41
- Appearance/Colour:beige powder
- Vapor Pressure:0.73Pa at 20-50℃
- Melting Point:140-142°C
- Boiling Point:1081.8 °C at 760 mmHg
- Flash Point:608 °C
- PSA:342.90000
- Density:1.955 at 20℃
- LogP:-0.59060
beta-Nicotinamide adenine dinucleotide disodium salt(Cas 606-68-8) Usage
|
Definition
|
A dehydrogenase complex that is the reduced formof NAD.
|
|
General Description
|
β-Nicotinamide adenine dinucleotide (β-NAD) regulates energy metabolism and immunity. It is a cofactor for mitochondrial deacetylase sirtuin-3 enzyme and modulates inflammasome assembly. β-NAD supresses interleukin-1β levels in monocytic cells in inflammatory syndromes. β-NAD released by neurosecretory cells is a potential neurotransmitter. β-NAD is a vascular mediator in lung endothelial cells and may play a protective role against cytokine mediated inflammation.
|
|
Biological Activity
|
NADH is a coenzyme that functions as a regenerating electron donor in catabolic processes including glycolysis, β-oxidation and the citric acid cycle (Krebs cycle, TCA cycle). It participates in cell signaling events as well, for example as a substrate for the poly (ADP-ribose) polymerases (PARPs) during the DNA damage response. The NAD+/NADH dependent sirtuins play key roles in stress responses during events involving energy metabolism, with implications in cancer biology, diabetes and neurodegenerative disease.As a reagent, NADH can be used in enzyme cycling assays to amplify detection of activity of biologically relevant enzymes or metabolites present in low concentrations.
|
|
Biochem/physiol Actions
|
NADH is a coenzyme that functions as a regenerating electron donor in catabolic processes including glycolysis, β-oxidation and the citric acid cycle (Krebs cycle, TCA cycle). It participates in cell signaling events as well, for example as a substrate for the poly (ADP-ribose) polymerases (PARPs) during the DNA damage response. The NAD+/NADH dependent sirtuins play key roles in stress responses during events involving energy metabolism, with implications in cancer biology, diabetes and neurodegenerative disease.As a reagent, NADH can be used in enzyme cycling assays to amplify detection of activity of biologically relevant enzymes or metabolites present in low concentrations.
|
|
Biotechnological Applications
|
Reduced β-nicotinamide adenine dinucleotide (NADH) plays a major role in metabolism as a cofactor in redox reactions and as a mobile electron carrier. NADH is a high energy compound that donates electrons to the electron transport chain to provide energy for ATP production by oxidative phosphorylation. NADH is a required oxidizing cosubstrate in fermentation, which regenerates NAD. NADH is fluorescent, which provides for a relatively simple way to detect NADH in biological samples. NADH is also used in enzyme cycling assays to detect relevant biological molecules in tissues.
|
|
Purification Methods
|
This coenzyme is available in high purity, and it is advisable to buy a fresh preparation rather than to purify an old sample as purification will invariably lead to a more impure sample contaminated with the oxidised form (NAD). It has UV max at 340nm ( 6,200 M-1cm-1) at which wavelength the oxidised form NAD has no absorption. At 340nm a 0.161mM solution in a 1cm (pathlength) cell has an absorbance of 1.0 unit. The purity is best checked by the ratio A280nm/A340nm ~2.1, a value which increases as oxidation proceeds. The dry powder is stable indefinitely at -20o. Solutions in aqueous buffers at pH ~7 are stable for extended periods at -20o and for at least 8hours at 0o, but are oxidised more rapidly at 4o in a cold room (e.g. almost completely oxidised overnight at 4o). [UV: Drabkin J Biol Chem 175 563 1945, Fluorescence: Boyer & Thorell Acta Chem Scand 10 447 1956, Redox: Rodkey J Biol Chem 234 188 1959, Schlenk in The Enzymes 2 250, 268 1951, Kaplan in The Enzymes 3 105, 112 1960.] Deuterated NADH, i.e. NADD, has been purified through the anion exchange resin AG-1 x 8 (100-200 mesh, formate form) and through a Bio-Gel P-2 column. [Viola et al. Anal Biochem 96 334 1979, Beilstein 26 III/IV 3639.]
|
InChI:InChI=1/C21H27N7O14P2.2Na/c22-17-12-19(25-7-24-17)28(8-26-12)21-16(32)14(30)11(41-21)6-39-44(36,37)42-43(34,35)38-5-10-13(29)15(31)20(40-10)27-3-1-2-9(4-27)18(23)33;;/h1-4,7-8,10-11,13-16,20-21,29-32H,5-6H2,(H5-,22,23,24,25,33,34,35,36,37);;/q;2*+1/p-1/t10-,11-,13-,14-,15-,16-,20-,21-;;/m1../s1
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego