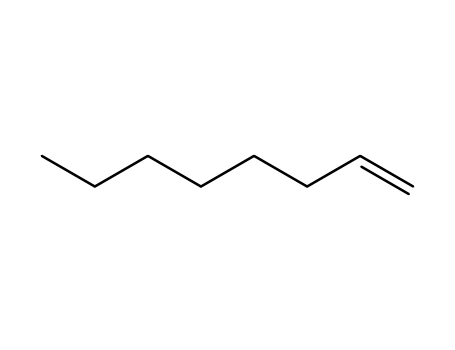
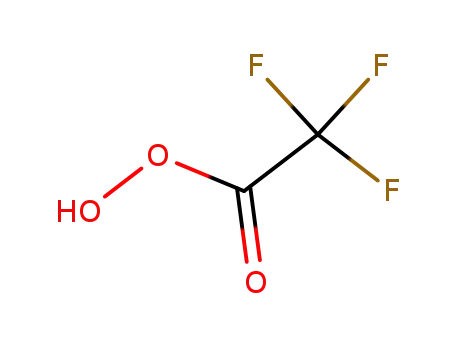
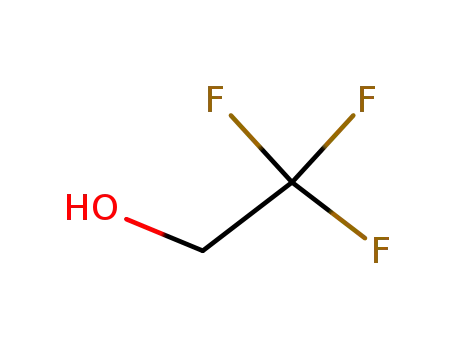
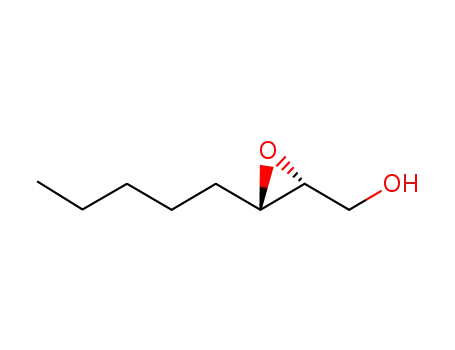
Epoxidation of alkenes catalyzed by some molybdenum(0) and molybdenum(IV) complexes
Acharya, Sitaram,Hanna, Tracy A.
, p. 113 - 123 (2016)
Catalytic epoxidations of styrene, cyclo...
Nonheme manganese-catalyzed asymmetric oxidation. A lewis acid activation versus oxygen rebound mechanism: Evidence for the "third oxidant"
Ottenbacher, Roman V.,Bryliakov, Konstantin P.,Talsi, Evgenii P.
, p. 8620 - 8628 (2010)
The catalytic properties of a series of ...
Rate-determining water-assisted O-O bond cleavage of an Fe III-OOH intermediate in a bio-inspired nonheme iron-catalyzed oxidation
Oloo, Williamson N.,Fielding, Andrew J.,Que, Lawrence
, p. 6438 - 6441 (2013)
Hydrocarbon oxidations by bio-inspired n...
Cerium(IV)-catalyzed deprotection of acetals and ketals under mildly basic conditions
Marko, Istvan E.,Ates, Ali,Gautier, Arnaud,Leroy, Bernard,Plancher, Jean-Marc,Quesnel, Yannick,Vanherck, Jean-Christophe
, p. 3207 - 3209 (1999)
-
Iron-catalyzed olefin cis-dihydroxylation by H2O2: Electrophilic versus nucleophilic mechanisms
Fujita, Megumi,Costas, Miquel,Que Jr., Lawrence
, p. 9912 - 9913 (2003)
Previous studies have classified a serie...
-
Barchi,J.J.,Moore,R.E.,Patterson,G.M.
, p. 8193 (1984)
-
OsO(4) in ionic liquid [Bmim]PF(6): a recyclable and reusable catalyst system for olefin dihydroxylation. remarkable effect of DMAP.
Yao, Qingwei
, p. 2197 - 2199 (2002)
[reaction: see text] The combination of ...
Ligand topology effects on olefin oxidations by bio-inspired [Fe II(N2Py2)] catalysts
Mas-Balleste, Ruben,Costas, Miquel,Van Den Berg, Tieme,Que Jr., Lawrence
, p. 7489 - 7500 (2006)
Linear tetradentate N2Py2 ligands can co...
Access to enantiopure aromatic epoxides and diols using epoxide hydrolases derived from total biofilter DNA
Kotik, Michael,Stepanek, Vaclav,Grulich, Michal,Kyslik, Pavel,Archelas, Alain
, p. 41 - 48 (2010)
Metagenomic DNA is a rich source of gene...
Total synthesis of the salicyldehydroproline-containing antibiotic promysalin
Kaduskar, Rahul D.,Dhavan, Atul A.,Dallavalle, Sabrina,Scaglioni, Leonardo,Musso, Loana
, p. 2034 - 2041 (2016)
A convergent total synthesis of promysal...
Iron-catalyzed olefin cis-dihydroxylation using a bio-inspired N,N, O-ligand
Oldenburg, Paul D.,Shteinman, Albert A.,Que Jr., Lawrence
, p. 15672 - 15673 (2005)
Nature has evolved enzymes that carry ou...
Dendrimer crown-ether tethered multi-wall carbon nanotubes support methyltrioxorhenium in the selective oxidation of olefins to epoxides
Bizzarri, Bruno Mattia,Botta, Lorenzo,Crucianelli, Marcello,Fanelli, Angelica,Ferella, Francesco,Gontrani, Lorenzo,Sadun, Claudia,Saladino, Raffaele
, p. 17185 - 17194 (2020)
Benzo-15-crown-5 ether supported on mult...
Chemical activation of a mononuclear non-porphyrinic manganese complex using water as oxygen source for the oxygen atom transfer reaction
El Kadiri, Moulay Youness,El Ghachtouli, Sanae,Guillot, Regis,Billon, Laurianne,Charlot, Marie-France,Framery, Eric,Andrioletti, Bruno,Aukauloo, Ally
, p. 2147 - 2150 (2012)
Water, a powerful ally: A MnIII complex ...
Cis-Dihydroxylation of electron deficient olefins catalysed by an oxo-bridged diiron(III) complex with H2O2
Kejriwal, Ambica,Biswas, Sachidulal,Biswas, Achintesh N.,Bandyopadhyay, Pinaki
, p. 77 - 84 (2016)
Room temperature oxidation of olefins ca...
Direct Catalytic Transformation of Olefins into α-Hydroxy Ketones with Hydrogen Peroxide Catalyzed by Peroxotungstophosphate
Sakata, Yasuyuki,Katayama, Yuji,Ishii, Yasutaka
, p. 671 - 674 (1992)
Aliphatic olefins were directly converte...
OsO4-catalyzed dihydroxylation of olefins in ionic liquid [emim]BF4: A recoverable and reusable osmium
Yanada, Reiko,Takemoto, Yoshiji
, p. 6849 - 6851 (2002)
We have demonstrated the usefulness of r...
Catalytic Asymmetric Dihydroxylation of Aliphatic Olefins with Reusable Resin-Osmium Tetroxide
Choudary, Boyapati M.,Jyothi, Karangula,Madhi, Sateesh,Kantam, M. Lakshmi
, p. 1190 - 1192 (2003)
Asymmetric dihydroxylation of aliphatic ...
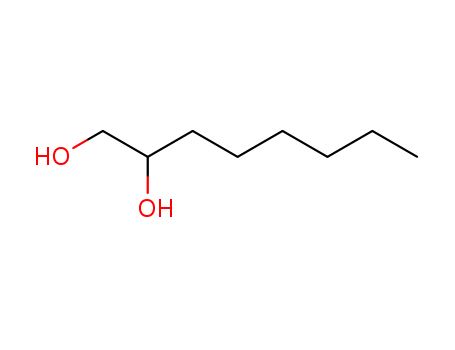
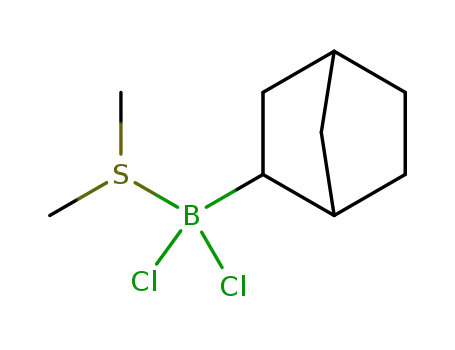
An original catalytic synthesis of boriran-1-ols
Khafizova, Leila O.,Khusainova, Liliya I.,Tyumkina, Tat'yana V.,Ryazanov, Kirill S.,Popod'ko, Natal'ya R.,Dzhemilev, Usein M.
, p. 577 - 578 (2018)
2-Alkylboriran-1-ols were obtained in a ...
Synthesis of di-nitrogen Schiff base complexes of methyltrioxorhenium(VII) and their application in epoxidation with aqueous hydrogen peroxide as oxidant
Gao, Yu,Zhang, Yuecheng,Qiu, Chuanjiang,Zhao, Jiquan
, p. 54 - 60 (2011)
Several di-nitrogen Schiff bases were sy...
Formation of persulphate from sodium sulphite and molecular oxygen catalysed by H5PV2Mo10O40-aerobic epoxidation and hydrolysis
Rubinstein, Amir,Carmeli, Raanan,Neumann, Ronny
, p. 13247 - 13249 (2014)
The H5PV2Mo10O40 polyoxometalate catalys...
Ligand topology tuning of iron-catalyzed hydrocarbon oxidations
Costas, Miquel,Que Jr., Lawrence
, p. 2179 - 2181 (2002)
Quite unexpectedly, the topology of the ...
The asymmetric ene reaction of N-glyoxyloyl-(2R)-bornane-10,2-sultam with 1-pentene and 1-hexene
Jezewski, Artur,Chajewska, Katarzyna,Wielogorski, Zbigniew,Jurczak, Janusz
, p. 1741 - 1749 (1997)
The asymmetric ene reaction of N-glyoxyl...
Niobium oxide and phosphoric acid impregnated silica-titania as oxidative-acidic bifunctional catalyst
Mohd Ekhsan, Jamilah,Lee, Siew Ling,Nur, Hadi
, p. 142 - 148 (2014)
Silica-titania modified by impregnation ...
Post-modified porphyrin imine gels with improved chemical stability and efficient heterogeneous activity in CO2 transformation
Liao, Peisen,Cai, Guangmei,Shi, Jianying,Zhang, Jianyong
, p. 10017 - 10024 (2019)
Efficient heterogeneous gel catalysts ha...
High conversion of olefins to cis-diols by non-heme iron catalysts and H2O2.
Ryu, Ju Yeon,Kim, Jinheung,Costas, Miquel,Chen, Kui,Nam, Wonwoo,Que Jr., Lawrence
, p. 1288 - 1289 (2002)
Efficient and highly stereoselective oxi...
Highly regio- and stereo-controlled Pd(0)-catalyzed nucleophilic substitution reaction for the synthesis of optically active γ-fluoroalkylated allylic alcohols
Konno,Ishihara,Yamanaka
, p. 8467 - 8472 (2000)
Pd(0)-catalyzed nucleophilic substitutio...
-
Emmons et al.
, p. 3472 (1954)
-
Oxidizing Ability of a Dioxygen-Activating Nonheme Iron(II)-Benzilate Complex Immobilized on Gold Nanoparticles
Sheet, Debobrata,Bera, Abhijit,Jana, Rahul Dev,Paine, Tapan Kanti
, p. 4828 - 4841 (2019)
An iron(II)-benzilate complex [(TPASH)Fe...
Raw and waste plant materials as sources of fungi with epoxide hydrolase activity. Application to the kinetic resolution of aryl and alkyl glycidyl ethers
Dolcet, Marta,Torres, Mercè,Canela-Garayoa, Ramon
, p. 78 - 88 (2018)
The by-products of olive oil production ...
Synthesis and characterization of binary and ternary oxovanadium complexes of N,N′-(2-pyridyl)thiourea and curcumin: Catalytic oxidation potential, antibacterial, antimicrobial, antioxidant and DNA interaction studies
Adam, Mohamed Shaker S.,Youssef, Magdy M.,Aboelghar, Maha F.,Hafez, Aly M.,El-Ayaan, Usama
, (2017)
Two binary and two ternary mono-oxovanad...
Iron-catalyzed olefin epoxidation in the presence of acetic acid: Insights into the nature of the metal-based oxidant
Mas-Balleste, Ruben,Que Jr., Lawrence
, p. 15964 - 15972 (2007)
The iron complexes [(BPMEN)Fe(OTf)2] (1)...
Synthesis of S-Methyl 2-Hydrxyalkanethioates, 2-Hydroxyalkanoic Acids and Related Compounds via the Addition Reaction of Tris(methylthio)methanide Ion to Alkanals
Orito, Kazuhiko,Seki, Yoshikatsu,Suginome, Hiroshi,Iwadare, Tsukasa
, p. 2013 - 2017 (1989)
In connection with the studies on biolog...
Iridium-promoted conversion of terminal epoxides to primary alcohols under acidic conditions using hydrogen
Rainsberry, Alena N.,Sage, Jarrod G.,Scheuermann, Margaret L.
, p. 3020 - 3022 (2019)
A strategy for the conversion of termina...
In situ formation of peracetic acid in iron-catalyzed epoxidations by hydrogen peroxide in the presence of acetic acid
Fujita, Megumi,Que Jr., Lawrence
, p. 190 - 194 (2004)
Iron complexes (tpa)Fe( OTf)2 (1) and (b...
Atom-efficient oxidation of alkenes with molecular oxygen: Synthesis of diols
Doebler, Christian,Mehltretter, Gerald,Beller, Matthias
, p. 3026 - 3028 (1999)
Dihydroxylations of simple alkenes were ...
A structural and functional model for dioxygenases with a 2-His-1-carboxylate triad
Oldenburg, Paul D.,Ke, Chun-Yen,Tipton, A. Alex,Shteinman, Albert A.,Que Jr., Lawrence
, p. 7975 - 7978 (2006)
(Chemical Equation Presented) An attract...
Absolute configuration of octanol derivatives in apple fruits
Beuerle, Till,Schreier, Peter,Brunerie, Pascal,Bicchi, Carlo,Schwab, Wilfried
, p. 145 - 149 (1996)
In extracts obtained by liquid liquid ex...
Enantioselective total synthesis of (+)-Hagen's gland lactones
Gharpure, Santosh J.,Nanda, Laxmi Narayan,Shukla, Manoj Kumar
, p. 6632 - 6635 (2011)
Enantioselective synthesis of Hagen's gl...
Enantioselective synthesis of terminal 1,2-diols from acyl chlorides
Shao, Panlin,Shen, Litao,Ye, Song
, p. 2688 - 2692 (2012)
Optically active terminal 1,2-diols were...
An osmium(III)/osmium(V) redox couple generating OsV(O)(OH) center for cis -1,2-dihydroxylation of alkenes with H2O2: Os complex with a nitrogen-based tetradentate ligand
Sugimoto, Hideki,Kitayama, Kazuhiro,Mori, Seiji,Itoh, Shinobu
, p. 19270 - 19280 (2012)
For the synthesis of the 1,2-diols, cis-...
A new practical method for the osmium-catalyzed dihydroxylation of olefins using bleach as the terminal oxidant
Mehltretter, Gerald M.,Bhor, Santosh,Klawonn, Markus,D?bler, Christian,Sundermeier, Uta,Eckert, Markus,Militzer, Hans-Christian,Beller, Matthias
, p. 295 - 301 (2003)
A general procedure for the osmium-catal...
Preparation of high purity 1,2-diols by catalytic oxidation of linear terminal alkenes with H2O2 in the presence of carboxylic acids under solvent-free conditions
Wu, Xu-Wei,Li, Bin-Dong
, p. 459 - 462 (2014)
The oxidation of terminal alkenes was sm...
Catalytic oxygen atom transfer promoted by tethered Mo(VI) dioxido complexes onto silica-coated magnetic nanoparticles
Colaiezzi, Roberta,Crucianelli, Marcello,Di Giuseppe, Andrea,Ferella, Francesco,Lazzarini, Andrea,Paolucci, Valentina
, (2021/11/30)
The preparation of three novel active an...
Structure-Guided Regulation in the Enantioselectivity of an Epoxide Hydrolase to Produce Enantiomeric Monosubstituted Epoxides and Vicinal Diols via Kinetic Resolution
Hou, Xiao-Dong,Hu, Bo-Chun,Hu, Die,Lei, Yu-Qing,Rao, Yi-Jian,Wu, Min-Chen,Zhang, Dong
supporting information, p. 1757 - 1761 (2022/03/16)
Structure-guided microtuning of an Asper...
A stand-alone cobalt bis(dicarbollide) photoredox catalyst epoxidates alkenes in water at extremely low catalyst load
Guerrero, Isabel,Romero, Isabel,Teixidor, Francesc,Vi?as, Clara
supporting information, p. 10123 - 10131 (2021/12/27)
The cobalt bis(dicarbollide) complex, Na...
Well-defined Cp*Co(III)-catalyzed Hydrogenation of Carbonates and Polycarbonates
Dahiya, Pardeep,Gangwar, Manoj Kumar,Sundararaju, Basker
, p. 934 - 939 (2020/12/15)
We herein report the catalytic hydrogena...
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego






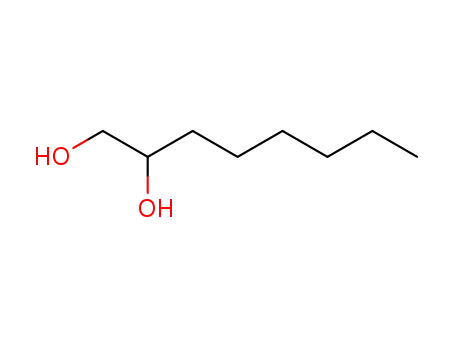
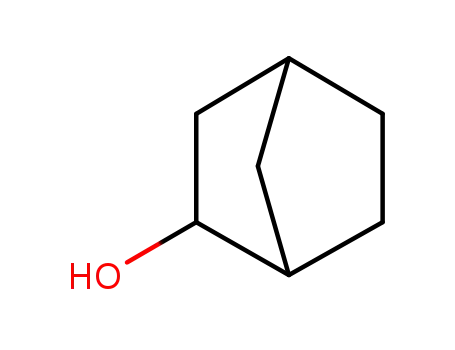
![1-( bicyclo[2.2.1]hept-2-yloxy)-2-hexylborirane](/upload/2024/5/7625524e-7048-47e9-a563-bd1d7922d92d.png)