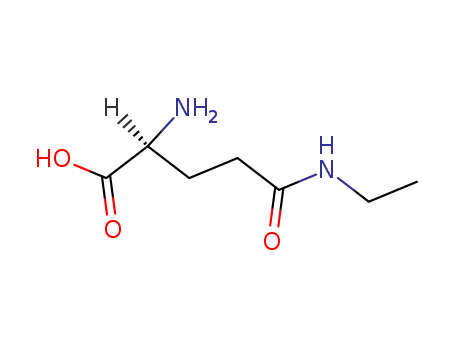
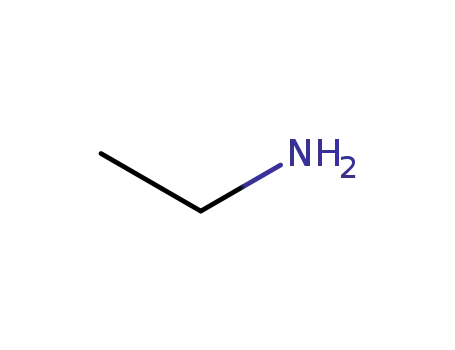
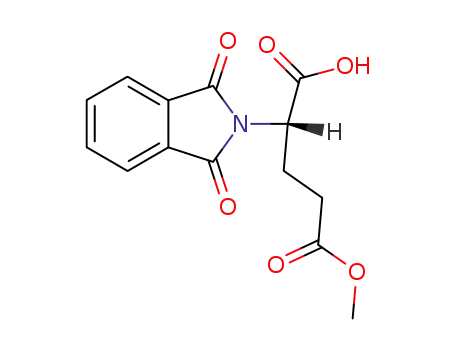
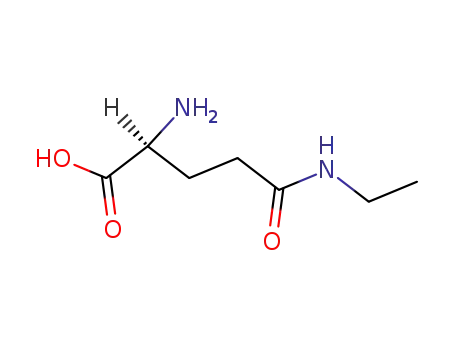
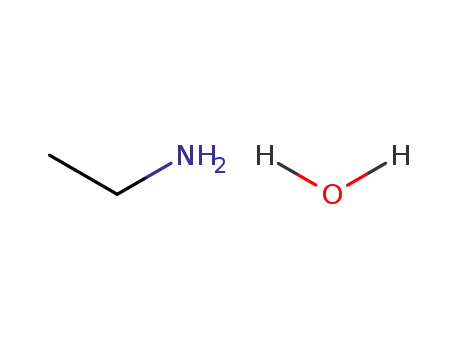
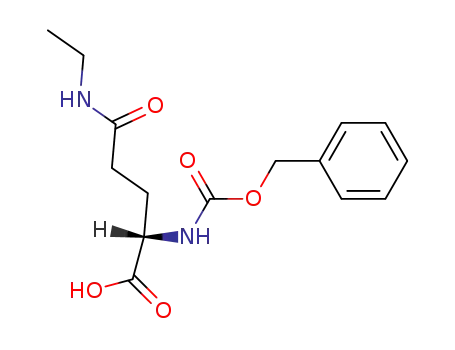
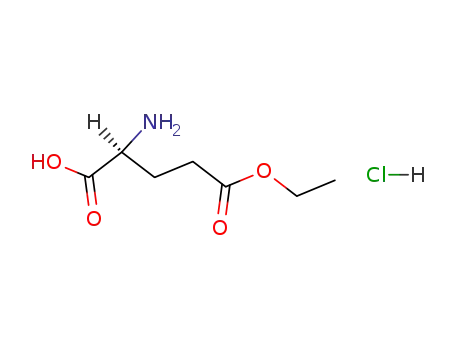
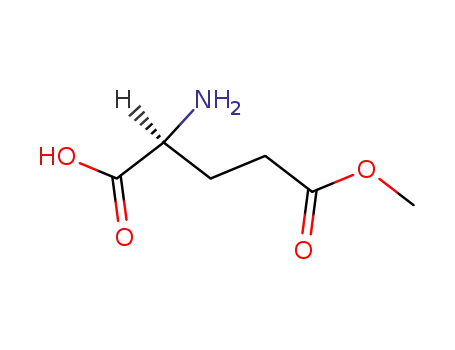
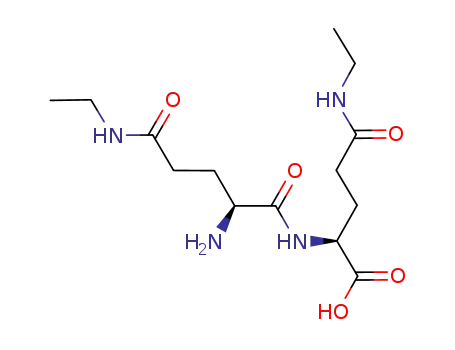
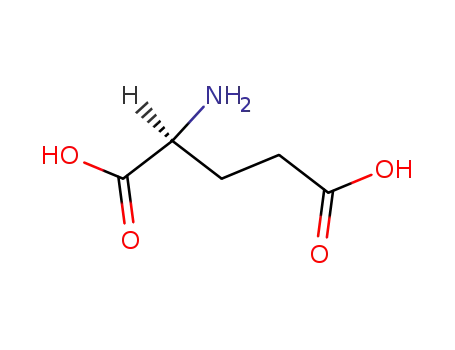
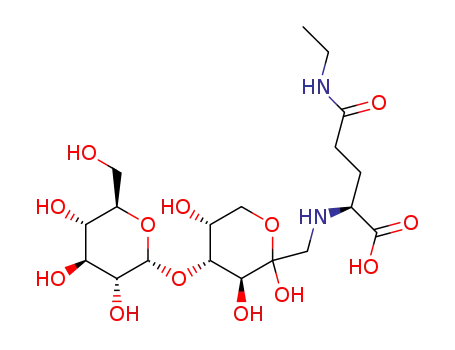
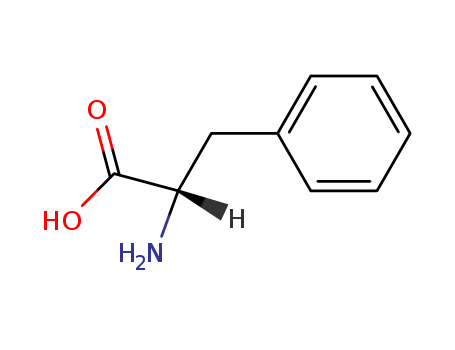
|
Reference
|
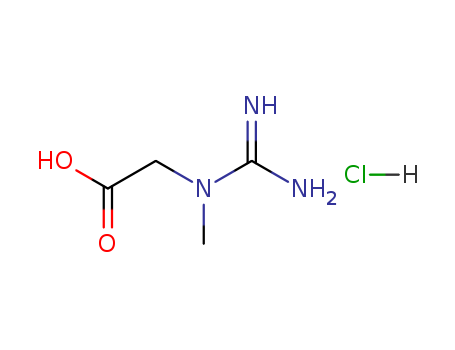
1 Sakato Y, The chemical constituents of tea: III. A new amide theanine. Nippon Nogei Kagakukaishi 23:262–267 [1949]. 2 Wan X, Zhang Z and Li D, Chemistry and biological properties of theanine, in Tea and Tea Products, ed.byHoCT, Lin JKandShahidi F. CRC Press, Boca Raton, pp. 255–274 [2009]. 3. G. Eschenauer, B.V. Sweet, Pharmacology and therapeutic uses of theanine, Am. J. Health Syst. Pharm. 63 [1][2006]28–30. 4. D.J. White, S. de Klerk, W. Woods, S. Gondalia, C. Noonan, A.B. Scholey, Antistress, behavioural and magnetoencephalography effects of an L-theanine-based nutrient drink: a randomised, double-blind, placebo-controlled, crossover trial, Nutrients 8 [1][2016]53. 5. ?M.S. Butt, R.S. Ahmad, M.T. Sultan, M.M.N. Qayyum, A. Naz, Green tea and anticancer perspectives: updates from last decade, Crit. Rev. Food Sci. Nutr. 55 [6][2015]792–805. 6. ?V. Crespy, G. Williamson, A review of the health effects of green tea catechins in in vivo animal models, J. Nutr. 134 [12][2004]3431–3440. 7. ?R. Cooper, Green tea and theanine: health benefits, Int. J. Food Sci. Nutr. 6 [3][2012]90–97. 8. ?Juneja LR, ChuDC, Okubo T,Nagato Y and Yokogoshi H, L-Theanine – a unique amino acid of green tea and its relaxation effect in humans. Trends Food Sci Technol 10:199–204 [1999]. 9. ?Balentine DA, Harbowy ME and Graham HN, Tea: The plant and its manufacture; chemistry and consumption of the beverage, in Caffeine, ed. by Spiller GA. CRC Press, Boca Raton, pp. 35–72 [1998]. 10. Chu DC, Green tea – its cultivation, processing of the leaves for drinking materials, and kinds of green tea, in Chemistry and Applications of Green Tea, ed. by Yamamoto T, Juneja LR, Chu DC and Kim M. CRC Press, Boca Raton, pp. 1–11 [1997]. 11. Deng WW, Ogita S and Ashihara H, Biosynthesis of theanine [γ ethylamino-l-glutamic acid]in seedlings of Camellia sinensis. Phytochem Lett 1:115–119 [2008]. 12. Chu DC, Kobayashi K, Juneja LR and Yamamoto T, Theanine – its synthesis, isolation, and physiological activity, in Chemistry and Applications of Green Tea, ed. by Yamamoto T, Juneja LR, Chu DC and Kim M. CRC Press, Boca Raton, pp. 129–135 [1997]. 13. Yamano HandMiyagawa K,Buffer capacity curves of green tea extracts using a personal computer with numerically treated online software. Food Sci Technol Int Tokyo 3:69–73 [2009]. 14. Ekborg-Ott KH, Taylor A and Armstrong DW, Varietal differences in the total and enantiomeric composition of theanine in tea. J Agric Food Chem 45:353–363 [1997]. 15. Vuong VQ, Golding JB, Nguyen M and Roach PD, Extraction and isolation of catechins from tea. J Sep Sci 33:3415–3428 [2010]. 16. Ishizu T, Tsutsumi H and Sato T, Interaction between gallocatechin gallate and caffeine in crystal structure of 1 : 2 and 2 : 2 complexes. Tetrahedron Lett 50:4121–4124 [2009]. 17. Liang H, Liang Y, Dong J, Lu J, Xu H and Wang H, Decaffeination of fresh green tea leaf [Camellia sinensis]byhotwater treatment. Food Chem 101:1451–1456 [2007]. 18 Narukawa M, Morita K and Hayshi Y, L-Theanine elicits an umami taste with inosine 5’-monophosphate. Biosci Biotechnol Biochem 72:3015–3017 [2008]. 19 de Araujo IET, Kringelbach ML, Rolls ET and Hobden P, Representation of umami taste in the human brain. J Neurophysiol 90:313–319 [2003]. 20 Y. Kim, K.L. Goodnera, J.D. Parkb, J. Choib, S.T. Talcott, Changes in antioxidant phytochemicals and volatile composition of Camellia sinensis by oxidation during tea fermentation, Food Chem. 129 [4][2011]1331–1342. 21 W.W. Deng, S. Ogita, H. Ashihara, Biosynthesis of theanine [γ-ethylamino-l-glutamic acid]in seedlings of Camellia sinensis, Phytochem. Lett. 1 [2][2008]115–159. 22 M. Narukawa, Y. Toda, T. Nakagita, Y. Hayashi, T. Misaka, L-Theanine elicits umami taste via the T1R1 + T1R3 umami taste receptor, Amino Acids 46 [6][2014]1583–1587. 23 D.C. Chu, Green tea – its cultivation, processing of the leaves for drinking materials, and kinds of green tea, in: T. Yamamoto, J.R. Lekh, D.C. Chu, M. Kim [Eds.], Chemistry and Applications of Green Tea, CRC Press, Boca Raton, 1997, pp. 1–11. 24 L.R. Juneja, D.C. Chu, T. Okubo, Y. Nagato, H. Yokogoshi, L-theanine—a unique amino acid of green tea and its relaxation effect in humans, Trend Food Sci. Technol. 10 [12][1999]425. 25 K. Boros, N. Jedlinszki, D. Csupor, Theanine and caffeine content of infusions prepared from commercial tea samples, Pharmacogn. Mag. 12 [45][2016]75–79. 26 J. Williams, J. Kellett, P.D. Roach, A. McKune, D. Mellor, J. Thomas, N. Naumovski, L-theanine as a functional food additive: its role in disease prevention and health promotion, Beverages 2 [13][2016], http://dx.doi.org/10. 3390/beverages2020013. 27 Q.V. Vuong, M.C. Bowyer, P.D. Roach, L-theanine: properties, synthesis and isolation from tea, J. Sci. Food Agric. 91 [11][2011]1931–1939. 28 Lichtenstein N, Preparation of c-alkylamides of glutamic acid. J Am Chem Soc 64:1021–1022 [1942]. 29 Kawagishi H and Sugiyama K, Facile and large-scale synthesis of L-theanine. Biosci Biotechnol Biochem 56:689 [1992]. 30 Yan SH, Dufour JP and Meurens M, Synthesis and characterization of highly pure theanine. J Tea Sci 23:99–104 [2003]. 31. Gu H, Jiang Y and Wang J, A practical synthesis of ethyl L-glutamine [L-theanine]. Org Prep Proced Int 36:182–185 [2004]. 32. Zhang F, Zheng QZ, Jiao QC, Liu JZ and Zhao GH, Enzymatic synthesis of theanine from glutamic acid γ -methyl ester and ethylamine by immobilized Escherichia coli cells with γ –glutamyltranspeptidase activity. Amino Acids 39:1177–1182 [2010]. 33. Miyake K and Kakita S, A novel catalytic ability of γ –glutamylcysteine synthetase of Escherichia coli and its application in theanine production. Biosci Biotechnol Biochem 73:2677–2683 [2009]. 34. Yamamoto S, Wakayama M and Tachiki T, Cloning and expression of Methylovorus mays No. 9 gene encoding γ –glutamylmethylamide synthetase: An enzyme usable in theanine formation by coupling with the alcoholic fermentation system of baker’s yeast. Biosci Biotechnol Biochem 72:101–109 [2008]. 35. Zhou X, Zhang Z, Jia X, Wu Y, Luo L and Yin Z, Mn2+ enhances theanine-forming activity of recombinant glutamine synthetase from Bacillus subtilis in Escherichia coli. World J Microb Biotechnol 24:1267–1272 [2008]. 36. Suzuki H, Izuka S, Miyakawa N and Kumagai H, Enzymatic production of theanine, an ‘umami’ component of tea, from glutamine and ethylamine with bacterial γ -glutamyltranspeptidase. Enzyme Microb Technol 31:884–889 [2002]. 37. Zhang F, Zheng QZ, Jiao QC, Liu JZ and Zhao GH, Synthesis of theanine from glutamic acid γ -methyl ester and ethylamine catalyzed by Escherichia coli having γ –glutamyltranspeptidase activity. Biotechnol Lett 32:1147–1150 [2010]. 38. G.W. Varilek, F. Yang, E.Y. Lee, W.J.S. deVilliers, J. Zhong, H.S. Oz, K.F. Westberry, C.J. McClain, Green tea polyphenol extract attenuates inflammation in interleukin-2-difeicient mice, a model of autoimmunity, J. Nutr. 131 [7][2001]2034–2039. 39. S.M. Chacko, P.T. Thambi, R. Kuttan, I. Nishigaki, Beneficial effects of green tea: a literature review, Chin. Med. 5 [2010]13. 40 Cooper R, Morr′e DJ and Morr′e DM, Medicinal benefits of green tea: Part I. Review of noncancer health benefits. J Altern Complement Med 11:521–528 [2005]. 41 Mason R, 200 mg of Zen: L-theanine boosts alpha waves, promotes alert relaxation. Altern Complement Ther 7:91–95 [2001]. 42. Nobre AC, Rao A and Owen GN, L-theanine, a natural constituent in tea, and its effect on mental state. Asia Pac J Clin Nutr 17:167–168 [2008]. 43. Lu K, Gray MA, Oliver C, Liley DT, Harrison BJ, Bartholomeusz CF, et al, The acute effects of L-theanine in comparison with alprazolam on anticipatory anxiety in humans. Hum Psychopharmacol Clin 19:457–465 [2004]. 44. Haskell CF, Kennedy DO, Milne AL, Wesnes KA and Scholey AB, The effects of L-theanine, caffeine and their combination on cognition and mood. Biol Psychol 77:113–122 [2008]. 45 Owen GN, Parnell H, Bruin EAD and Rycroft JA, The combined effects of L-theanine and caffeine on cognitive performance and mood. Nutr Neurosci 11:193–198 [2008]. 46. Di X, Yan J, Zhao Y, Zhang J, Shi Z, Chang Y, et al, L-Theanine protects the APP [Swedish mutation]transgenic SH-SY5Y cell against glutamate-induced excitotoxicity via inhibition of the NMDA receptor pathway. Neuroscience 18:778–786 [2010]. 47. Liu Q, Duan H, Luan J, Yagasaki K and Zhang G, Effects of theanine on growth of human lung cancer and leukemia cells as well as migration and invasion of human lung cancer cells. Cytotechnology 59:211–217 [2009]. 47. Friedman M, Mackey BE, Kim HJ, Lee IS, Lee KR, Lee SU, et al, Structure–activity relationships of tea compounds against human cancer cells. J Agric Food Chem 55:243–253 [2007]. 48. Sugiyama T and Sadzuka Y, Theanine, a specific glutamate derivative in green tea, reduces the adverse reactions of doxorubicin by changing the glutathione level. Cancer Lett 212:177–184 [2004]. 49. Sadzuka Y, Sugiyama T, Suzuki T and Sonobe T, Enhancement of the activity of doxorubicin by inhibition of glutamate transporter. Toxicol Lett 123:159–167 [2001]. 50. Yokogoshi H and Kobayashi M, Hypotensive effect of γ -glutamylmethylamide in spontaneously hypertensive rats. Life Sci 62:1065–1068 [1998]. 51. Rogers PJ, Smith JE, Heatherley SV and Pleydell-Pearce CW, Time for tea: Mood, blood pressure and cognitive performance effects of caffeine and theanine administered alone and together. Psychopharmacology 195:569–577 [2007]. 52. Kurihara S, Shibahara S, Arisaka H and Akiyama Y, Enhancement of antigen-specific immunoglobulin G production in mice by co-administration of L-cystine and L-theanine. J Vet Med Sci 69:1263–1270 [2007]. 53. Takagi Y, Kurihara S, Higashi N, Morikawa S, Kase T, Maeda A, et al, Combined administration of L-cystine and L-theanine enhances immune functions and protects against influenza virus infection in aged mice. J VetMed Sci 72:157–165 [2010].
|
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego