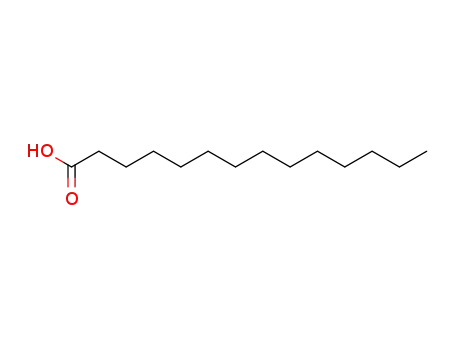|
Content Analysis
|
Weight 1.5 g sample. Then it is determined by the method ester assay (OT-18). The equivalent factor (e) in the calculation is 135.2. Or it is determined by a non-polar column method of gas chromatography (GT-10-4).
|
|
Production Method
|
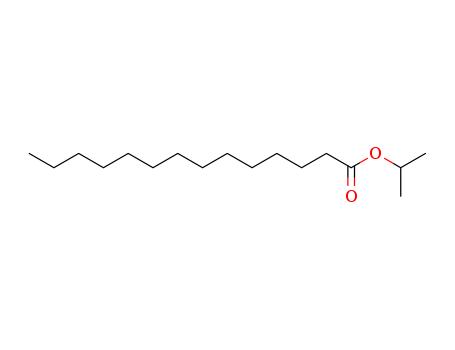
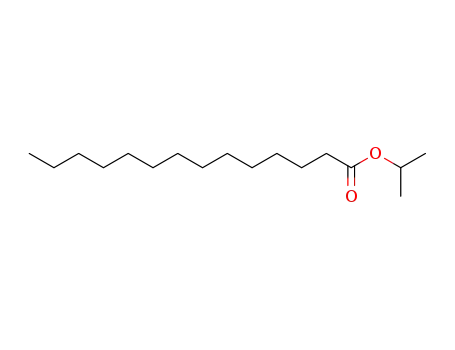
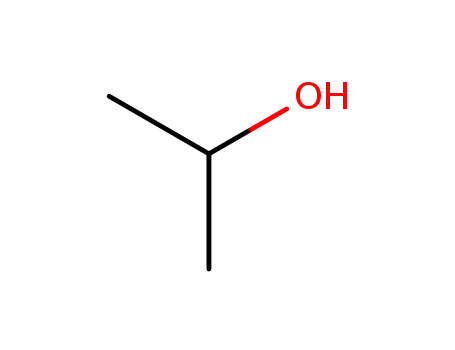
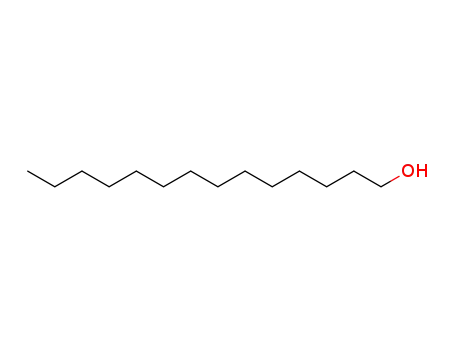
It is a product of esterification of myristic acid derived from re-steamed coconut coil with isopropyl alcohol. (1)? 200 kg myristic acid and 450 kg isopropyl alcohol were added into the reaction vessel in turn. After mixing, 360 kg sulfuric acid (98%) was added. The reaction mixture was heated to reflux for 10 hours. Isopropyl alcohol was then recovered, washed with ice water, and neutralized with Na2CO3 aqueous solution (10%). Under normal pressure, isopropyl alcohol and water were distilled. While under reduced pressure, isopropyl myristate was distilled (185°C/1.0kPa~195°C/2.7kPa). (2) 90 kg isopropyl alcohol was added into the reaction vessel and then sulfuric acid as catalyst, with 5% of the total amount, was added. During mixing, 228 kg myristic acid was added slowly. The mixture was heated to reflux and water was continuously separated. Until no water was separated, the reaction temperature was reduced and probe was obtained to measure the acid value. When the acid value reached 1.5 mg KOH/g, the reaction was completed. Alkali was then added for neutralization. After the removal of water under reduced pressure, the pressure was further reduced for dealcoholization until the acid value was 0.05~1.0 mg KOH/g. The final product is then isopropyl myristate.
|
|
Toxicity
|
ADI is not regulated (FAO/WHO, 2001).
|
|
Production Methods
|
Isopropyl myristate may be prepared either by the esterification of myristic acid with propan-2-ol or by the reaction of myristoyl chloride and propan-2-ol with the aid of a suitable dehydrochlorinating agent. A high-purity material is also commercially available, produced by enzymatic esterification at low temperature.
|
|
Preparation
|
By conventional esterification of isopropanol with myristic acid
|
|
Pharmaceutical Applications
|
Isopropyl myristate is a nongreasy emollient that is absorbed readily by the skin. It is used as a component of semisolid bases and as a solvent for many substances applied topically. Applications in topical pharmaceutical and cosmetic formulations include bath oils; make-up; hair and nail care products; creams; lotions; lip products; shaving products; skin lubricants; deodorants; otic suspensions; and vaginal creams. For example, isopropyl myristate is a self-emulsifying component of a proposed cold cream formula, which is suitable for use as a vehicle for drugs or dermatological actives; it is also used cosmetically in stable mixtures of water and glycerol. Isopropyl myristate is used as a penetration enhancer for transdermal formulations, and has been used in conjunction with therapeutic ultrasound and iontophoresis.It has been used in a water-oil gel prolonged-release emulsion and in various microemulsions. Such microemulsions may increase bioavailability in topical and transdermal applications. Isopropyl myristate has also been used in microspheres, and significantly increased the release of drug from etoposide-loaded microspheres. Isopropyl myristate is used in soft adhesives for pressuresensitive adhesive tapes.
|
|
Contact allergens
|
Despite wide use in cosmetics, perfumes, and topical medicaments, isopropyl myristate is a very weak sen- sitizer and a mild irritant.
|
|
Biochem/physiol Actions
|
Isopropyl myristate (myristic acid isopropyl ester) is used to change the physicochemical characteristics of microsheres such as poly(lactic-co-glycolic acid) (PLGA) microspheres. Isopropyl myristate is used as a oil phase component in the formulaton of microemulsion systems.
|
|
Pharmacology
|
Isopropyl myristate is used in pharmaceutical preparations because it improves solubility and increases absorption through the skin. External uses include a non-irritating iodine preparation for disinfecting the skin (Powers & Rieger, 1963) and aerosol bactericidal preparations for feminine hygiene use without irritation of the skin and mucous membranes (Geistlich, 1970; Watson. 1969). Preparations for internal use include oral steroid formulations (Hirata, 1970) and anaesthetic injection solutions (Davis, Pearce & Connor, 1972). Veterinary medications containing isopropyl myristate include oral or parenteral compositions for lungworm infections (N. V. Philips' Gloielampenfabrieken, 1964) and a spray formulation for bovine udders to treat mastitis, combat infection and improve the general skin condition (Kraus, 1965). Isopropyl myristate has been found to be an effective repository vehicle for im injection of penicillin in rabbits and for sc administration of oestrogens in ovariectomized rats (Platcow & Voss, 1954). In assays on human forearms, vasoconstrictor activity of ointment preparations containing 0025% betamethasone 17-benzoate in white soft paraffin was increased by the presence of isopropyl myristate (Pepler, Woodford & Morrison, 1971). Donovan, Ohmart & Stoklosa (1954) noted that the good solvent properties of isopropyl myristate might increase the therapeutic activity of formulations by the apparent alteration in particle size of the active ingredients, so that further evaluation and clinical study would be necessary before its use in extemporaneous compounding could be recommended. Studies in which the antifungal activity of paraben esters solubilized by surfactants was decreased by isopropyl myristate (Matsumoto & Aoki, 1962) indicate that the effectiveness of medicinal substances may be influenced by the presence of surfactants and oily ingredients such as isopropyl myristate.
|
|
Safety
|
Isopropyl myristate is widely used in cosmetics and topical pharmaceutical formulations, and is generally regarded as a nontoxic and nonirritant material. LD50 (mouse, oral): 49.7 g/kg LD50 (rabbit, skin): 5 g/kg
|
|
Metabolism
|
Higher molecular weight aliphatic esters are thought to be readily hydrolysed to the corresponding alcohols and acids which are then metabolized; isopropyl myristate is undoubtedly hydrolysed to normal metabolic products (Fassett, 1963). When myristic acid (as the ethyl ester) was fed to dogs,
|
|
storage
|
Isopropyl myristate is resistant to oxidation and hydrolysis, and does not become rancid. It should be stored in a well-closed container in a cool, dry place and protected from light.
|
|
Incompatibilities
|
When isopropyl myristate comes into contact with rubber, there is a drop in viscosity with concomitant swelling and partial dissolution of the rubber; contact with plastics, e.g. nylon and polyethylene, results in swelling. Isopropyl myristate is incompatible with hard paraffin, producing a granular mixture. It is also incompatible with strong oxidizing agents.
|
|
Regulatory Status
|
Included in the FDA Inactive Ingredients Database (otic, topical, transdermal, and vaginal preparations). Used in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
|
|
Brand name
|
Estergel (Merck).
|
|
General Description
|
Isopropyl myristate is an ester of isopropyl alcohol myristic acid. It is mainly used as a solubilizer, emulsifier and emollient in cosmetic and topical medicines. It also finds applications as a flavoring agent in the food industry.Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
|
|
Who Evaluation
|
Evaluation year: 1998
|
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego