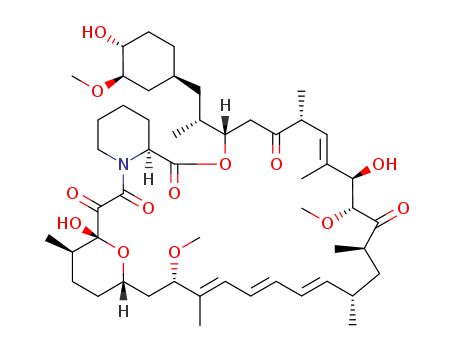
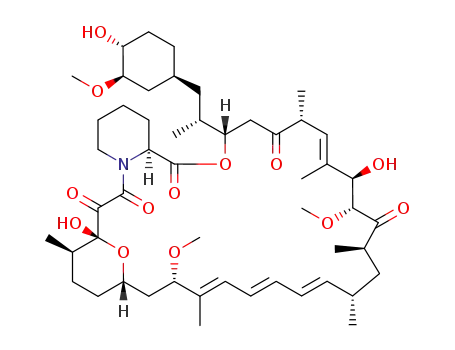
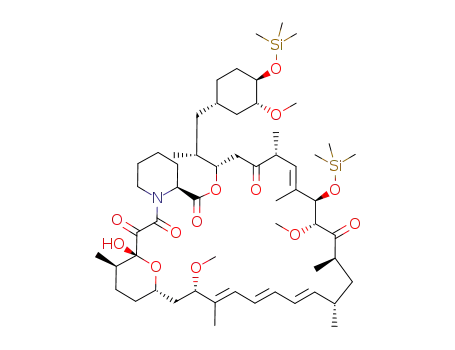
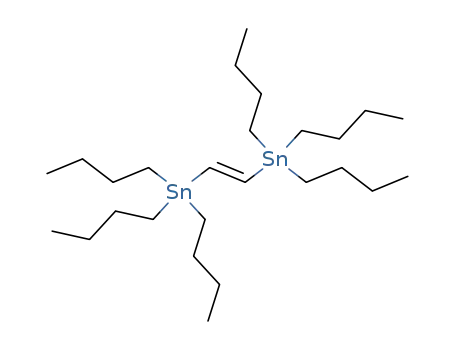
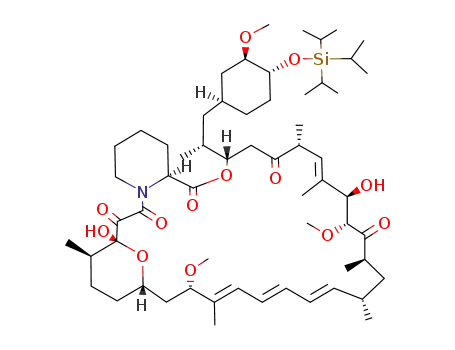
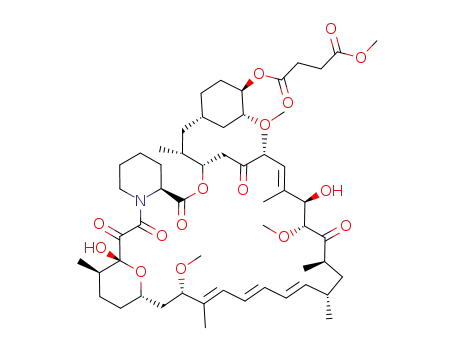
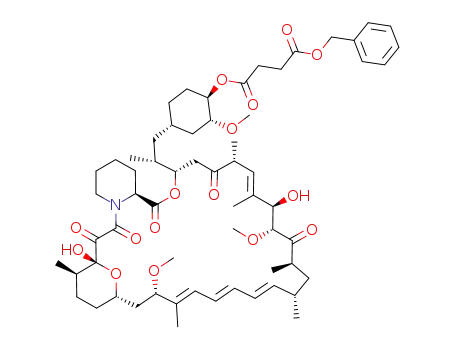
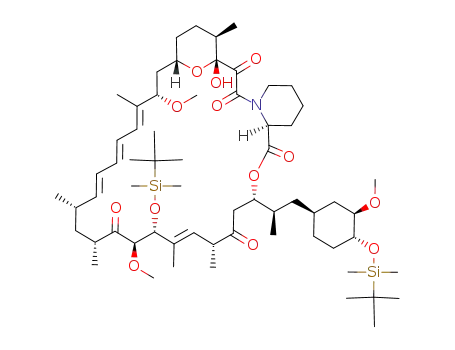
Top Quality Rapamycin 53123-88-9 Hot Sell In Stock
- Molecular Formula:C51H79NO13
- Molecular Weight:914.187
- Appearance/Colour:white to off-white solid
- Vapor Pressure:0mmHg at 25°C
- Melting Point:183-185 ºC
- Refractive Index:1.55
- Boiling Point:973.017 ºC at 760 mmHg
- PKA:10.40±0.70(Predicted)
- Flash Point:542.261 ºC
- PSA:195.43000
- Density:1.182 g/cm3
- LogP:6.11850
Rapamycin(Cas 53123-88-9) Usage
|
in vivo
|
Rapamycin, also known as Sirolimus, is a macrolide compound produced by the bacterium Streptomyces hygroscopicus, first discovered in soil samples from Easter Island in 1975. It has potent immunosuppressive and antifungal properties and is widely used in organ transplantation to prevent rejection. Rapamycin appears as a white solid crystal with a melting point of 183-185°C, and it is soluble in organic solvents like methanol, ethanol, acetone, and chloroform, but insoluble in water. |
|
Description
|
Rapamycin inhibits the mammalian target of rapamycin (mTOR), a key protein involved in cell growth and proliferation, making it useful for immunosuppression, cancer therapy, and potentially anti-aging treatments. It is marketed under the brand name Rapamune (Wyeth). Shanghai Wibson Biotechnology Co., Ltd. is a high-tech enterprise that mainly develops and sells pharmaceutical and chemical raw materials and other related products. The company has advanced and perfect supporting system and has formed advanced chemical synthesis technology, which can quickly meet the personalized needs and series development from suitable testing to industrial production. Products are exported to Russia, Southeast Asia and other countries, in the world has a very high reputation and visibility. The company has strong financial strength and a good operating environment, can provide customers with high-quality, efficient and fast services. |
| Manufacturing Process |
Rapamycin is produced through fermentation of Streptomyces hygroscopicus. The manufacturing process can be outlined in two main stages:
First-stage Inoculum:
Streptomyces hygroscopicus spores are inoculated in a medium containing peptone, sodium chloride, yeast extract, and glucose.
Incubated at 28°C on a shaker for 30 hours.
Production Stage:
A larger fermenter (250-liter) is used, with a production medium including soluble starch, ammonium sulfate, glucose, molasses, and other minerals.
The fermentation lasts five days at 28°C, after which the mixture is extracted with butanol and methanol to yield crude Rapamycin.
|
| Therapeutic Uses |
Rapamycin is approved for preventing organ rejection in kidney transplant patients. Unlike other immunosuppressants, it has no nephrotoxicity, making it safer for long-term use. Rapamycin and its analogs (rapalogs), such as everolimus and temsirolimus, are used in treating various cancers, including renal cell carcinoma, lymphoma, and neuroendocrine tumors. They target the mTOR pathway, which is overactive in many cancers. Rapamycin is FDA-approved for treating lymphangioleiomyomatosis (LAM), a rare lung disease, and shows promise for treating vascular anomalies and tuberous sclerosis complex (TSC). In addition to its use in transplantation and cancer treatment, Rapamycin-coated stents prevent coronary restenosis by limiting tissue overgrowth. It may also treat severe uveitis and age-related macular degeneration. |
InChI:InChI=1/C51H79NO13/c1-30-16-12-11-13-17-31(2)42(61-8)28-38-21-19-36(7)51(60,65-38)48(57)49(58)52-23-15-14-18-39(52)50(59)64-43(33(4)26-37-20-22-40(53)44(27-37)62-9)29-41(54)32(3)25-35(6)46(56)47(63-10)45(55)34(5)24-30/h11-13,16-17,25,30,32-34,36-40,42-44,46-47,53,56,60H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12-,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38?,39+,40-,42+,43+,44-,46-,47+,51-/m1/s1
53123-88-9 Relevant articles
Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease
Vivek Panwar, Aishwarya Singh, Manini Bhatt, Rajiv K. Tonk, Shavkatjon Azizov, Agha Saquib Raza, Shinjinee Sengupta, Deepak Kumar & Manoj Garg
, Signal Transduction and Targeted Therapy volume 8, Article number: 375 (2023)
The mammalian target of rapamycin (mTOR) is a protein kinase that controls cellular metabolism, catabolism, immune responses, autophagy, survival, proliferation, and migration, to maintain cellular homeostasis. The mTOR signaling cascade consists of two distinct multi-subunit complexes named mTOR complex 1/2 (mTORC1/2). mTOR catalyzes the phosphorylation of several critical proteins like AKT, protein kinase C, insulin growth factor receptor (IGF-1R), 4E binding protein 1 (4E-BP1), ribosomal protein S6 kinase (S6K),
Randomized, double-blind, placebo-controlled trial of rapamycin in amyotrophic lateral sclerosis
Jessica Mandrioli, Roberto D’Amico, Elisabetta Zucchi, Sara De Biasi, Federico Banchelli, Ilaria Martinelli, Cecilia Simonini, Domenico Lo Tartaro, Roberto Vicini, Nicola Fini, Giulia Gianferrari, Marcello Pinti, Christian Lunetta, Francesca Gerardi, Claudia Tarlarini, Letizia Mazzini, Fabiola De Marchi, Ada Scognamiglio, Gianni Sorarù, Andrea Fortuna, Giuseppe Lauria, Eleonora Dalla Bella, Claudia Caponnetto, Giuseppe Meo, …Andrea Cossarizza
, Nature Communications volume 14, Article number: 4970 (2023)
Of the secondary outcomes, rapamycin decreased mRNA relative expression of the pro-inflammatory cytokine IL-18, reduced plasmatic IL-18 protein, and increased the percentage of classical monocytes and memory switched B cells, although no corrections were applied for multiple tests. In conclusion, we show that rapamycin treatment is well tolerated and provides reassuring safety findings in ALS patients, but further trials are necessary to understand the biological and clinical effects of this drug in ALS.
53123-88-9 Process route
-
- 331810-10-7
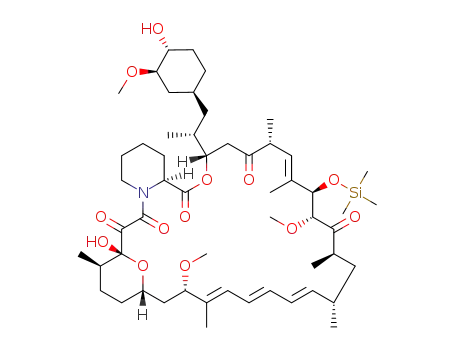
31-(trimethylsilylether)rapamycin
-
- 1111-92-8
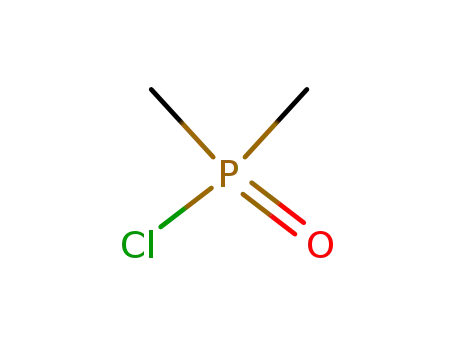
dimethylphosphinic acid chloride
-
- 572924-54-0
Ridaforolimus
Conditions
| Conditions |
Yield |
|
31-(trimethylsilylether)rapamycin; With 3,5-Lutidine; In dichloromethane; at 0 - 5 ℃; for 1.08333h; Inert atmosphere;
dimethylphosphinic acid chloride; In dichloromethane; at 0 - 5 ℃; for 1h; Inert atmosphere;
|
81.03 %Chromat.
5.14 %Chromat. |
-
- 331810-13-0
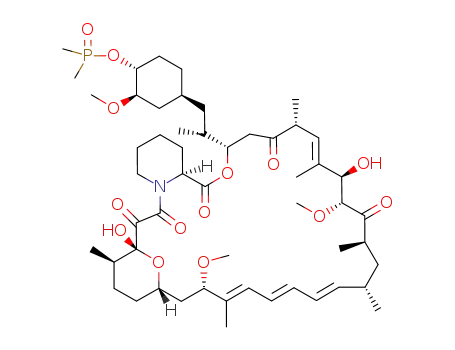
31,42-bis(trimethylsilylether)rapamycin
Conditions
| Conditions |
Yield |
|
C57H95NO13Si2; 31,42-bis(trimethylsilylether)rapamycin; With sulfuric acid; water; In acetone; at 0 - 5 ℃; for 0.166667h;
With sodium hydrogencarbonate; acetic acid; In water; acetone; at 0 - 15 ℃; for 0.283333 - 0.416667h;
With sodium acetate; In water; acetone; at 20 ℃; for 60.1667h; pH=5 - 5.5; Aqueous sodium acetate buffer;
|
87.6% |
53123-88-9 Upstream products
-
14275-61-7
(E)-1,2-bis(tri-n-butylstannyl)ethene
-
151122-98-4
C60H99NO13Si
-
155589-15-4
rapamycin 42-hemisuccinate methyl ester
-
143136-12-3
rapamycin 42-hemisuccinate benzyl ester
53123-88-9 Downstream products
-
156472-71-8
C63H107NO13Si2
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego