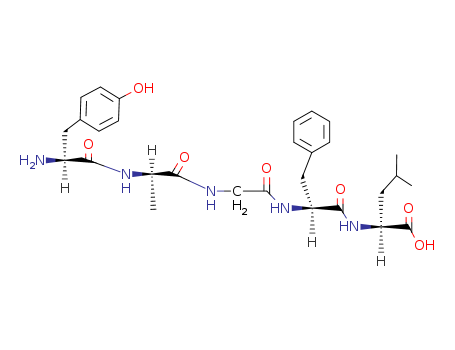
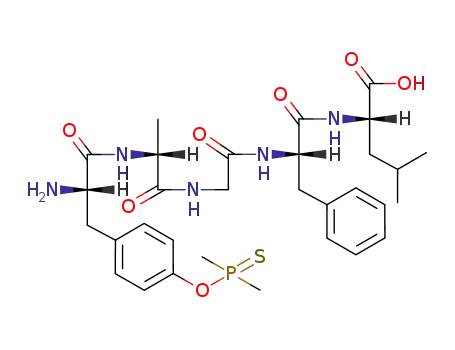
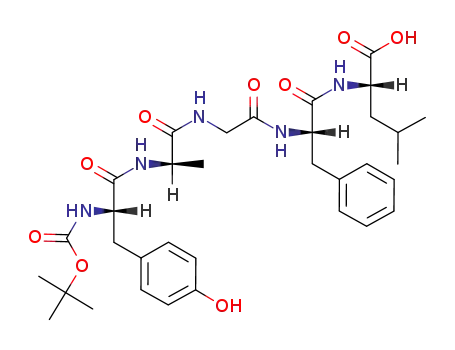
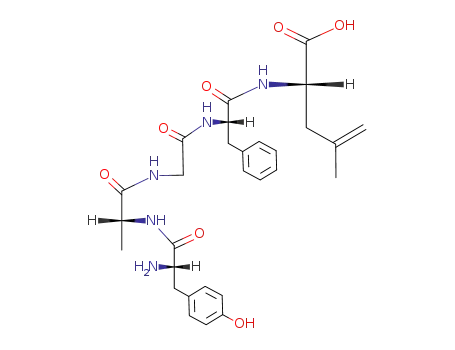
Buy Quality (D-Ala2)-Leu-Enkephalin 64963-01-5 In Stock with Immediately Delivery
- Molecular Formula:C29H39N5O7
- Molecular Weight:569.658
- Vapor Pressure:0mmHg at 25°C
- Melting Point:160-163.5 °C
- Refractive Index:1.582
- Boiling Point:991.9 °C at 760 mmHg
- PKA:3.40±0.10(Predicted)
- Flash Point:553.7 °C
- PSA:199.95000
- Density:1.257 g/cm3
- LogP:2.48980
(D-Ala2)-Leu-Enkephalin(Cas 64963-01-5) Usage
|
General Description
|
(D-Ala²)-Leu-Enkephalin is a synthetic peptide analog of the naturally occurring enkephalin, a type of endogenous opioid peptide that binds to opioid receptors in the brain. Enkephalins play a role in modulating pain and stress responses in the body. In this analog, the second amino acid in the sequence is modified, replacing glycine with D-alanine, which increases its stability against enzymatic degradation, prolonging its biological activity. Like natural enkephalins, (D-Ala²)-Leu-Enkephalin has potent analgesic effects by mimicking the action of endorphins, and it interacts primarily with delta-opioid receptors. Its structure includes five amino acids: D-alanine, tyrosine, glycine, phenylalanine, and leucine. |
InChI:InChI=1/C29H39N5O7/c1-17(2)13-24(29(40)41)34-28(39)23(15-19-7-5-4-6-8-19)33-25(36)16-31-26(37)18(3)32-27(38)22(30)14-20-9-11-21(35)12-10-20/h4-12,17-18,22-24,35H,13-16,30H2,1-3H3,(H,31,37)(H,32,38)(H,33,36)(H,34,39)(H,40,41)/t18-,22+,23+,24+/m1/s1
Shanghai Wibson Biotechnology Co., Ltd. is a high-tech enterprise that mainly develops and sells pharmaceutical and chemical raw materials and other related products. The company has advanced and perfect supporting system and has formed advanced chemical synthesis technology, which can quickly meet the personalized needs and series development from suitable testing to industrial production. Products are exported to Russia, Southeast Asia and other countries, in the world has a very high reputation and visibility. The company has strong financial strength and a good operating environment, can provide customers with high-quality, efficient and fast services.
64963-01-5 Relevant articles
Synthesis and antinociceptive activity of [D-Ala2]Leu-enkephalin derivatives conjugated with the adamantane moiety
Kitagawa, Kouki,Mizobuchi, Noriko,Hama, Teruo,Hibi, Tohru,Konishi, Ryoji,Futaki, Shiroh
, p. 1782 - 1787 (2007/10/03)
Based on the physicochemical and pharmac...
Synthesis of 2>Leu-enkephalin and 2,D-Leu5>Leu-enkephalin with High Specific Tritiated Activity in the Leucine Residue
Hasegawa, Hiroshi,Shinohara, Yoshihiko,Baba, Shigeo
, p. 2641 - 2644 (2007/10/02)
2>Leu-enkephalin and 2,D-Leu5>Leu-enkep...
A photoaffinity reagent to label the opiate receptors of guinea pig ileum and mouse vas deferens
Fujioka,Matsunaga,Nakayama,Kanaoka,Hayashi,Kangawa,Matsuo
, p. 836 - 840 (2007/10/02)
An enkephalin derivative, [D-Ala2,Leu5]e...
Phosphinyl- and Phosphinothioylamino Acids and Peptides. VI. The Protection of the Hydroxyl Function in the Tyrosine Side-chain by the Dimethylphosphinothioyl Group
Ueki, Masaaki,Inazu, Toshiyuki
, p. 204 - 207 (2007/10/02)
The use of the dimethylphosphinothioyl (...
64963-01-5 Process route
-
-
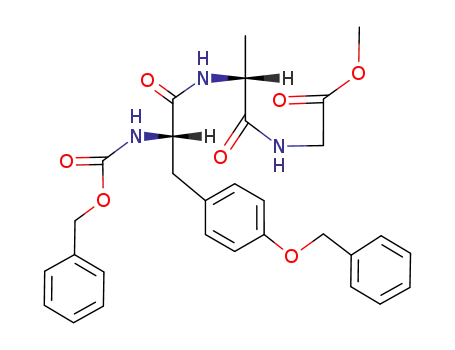
H-L-Tyr(Mpt)-D-Ala-Gly-L-Phe-L-Leu-OH
-
- 60284-47-1,63631-40-3,64963-01-5,64963-05-9,64963-08-2,64975-66-2
D-Ala2-Leu5-enkephalin
Conditions
| Conditions |
Yield |
|
With sodium hydroxide; In methanol; at 0 ℃;
|
94% |
-
- 73965-71-6
Nα-(tert-butoxycarbonyl)-2,Leu5>enkephalin
-
- 60284-47-1,63631-40-3,64963-01-5,64963-05-9,64963-08-2,64975-66-2
D-Ala2-Leu5-enkephalin
Conditions
| Conditions |
Yield |
|
With methoxybenzene; trifluoroacetic acid;
|
47% |
64963-01-5 Upstream products
-
131124-60-2
2,4,5-didehydro-L-Leu5>Leu-enkephalin
-
73965-71-6
Nα-(tert-butoxycarbonyl)-2,Leu5>enkephalin
-
86827-18-1
N-benzyloxycarbonyl-O-benzyl-L-tyrosine
-
139143-40-1
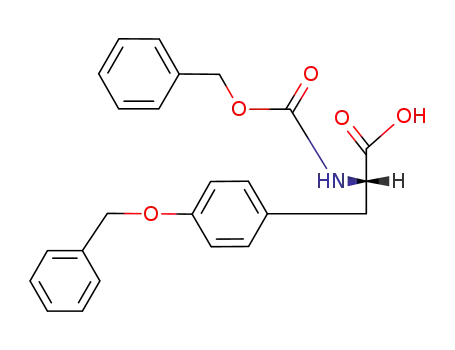
{(R)-2-[(S)-2-Benzyloxycarbonylamino-3-(4-benzyloxy-phenyl)-propionylamino]-propionylamino}-acetic acid methyl ester
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego