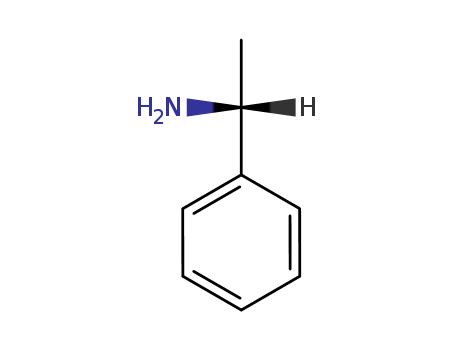
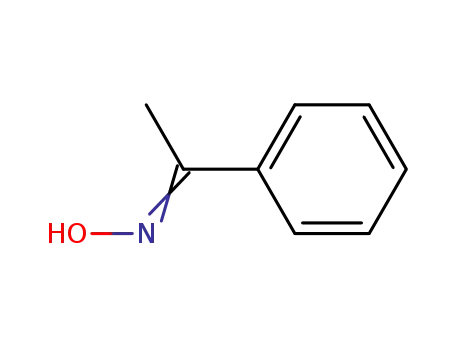
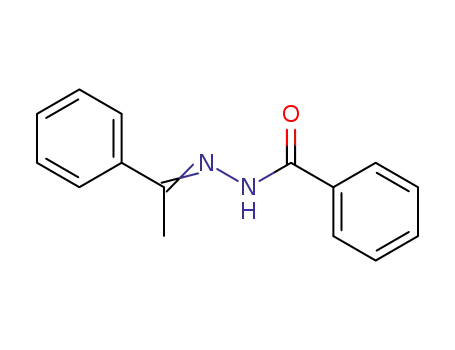
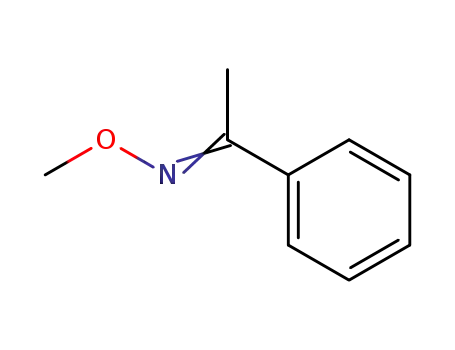
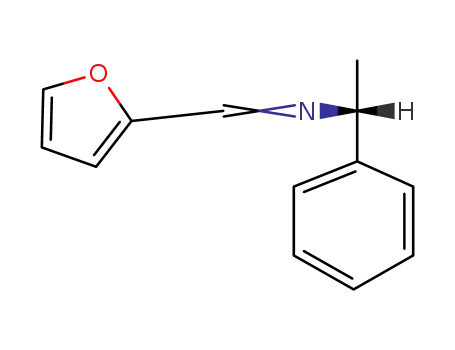
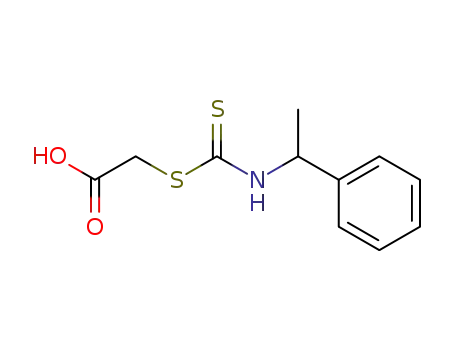
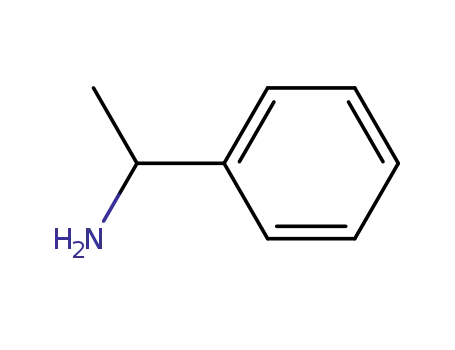Synthesis and reactivity towards carbon monoxide of an optically active endo five-membered ortho-cyclopalladated imine: X-ray molecular structure of trans-(μ-Cl)2[Pd(κ2-C,N-(R)-C6H4-CH{double bond, long}N-CHMe-Ph)]2
Albert, Joan,D'Andrea, Luci?a,Granell, Jaume,Tavera, Raquel,Font-Bardia, Mercè,Solans, Xavier
, p. 3070 - 3080 (2007)
(R)-1-Phenylethyl-benzylidene-amine (1) ...
Crystallization-based resolution of 1,4-benzodioxane-2-carboxylic acid enantiomers via diastereomeric 1-phenylethylamides
Fumagalli, Laura,Bolchi, Cristiano,Bavo, Francesco,Pallavicini, Marco
, p. 2009 - 2011 (2016)
Unlike the diastereomeric 1-phenylethyla...
Determination of absolute configurations of amines and amino acids using nonchiral derivatizing agents (NCDA) and deuterium NMR.
Chalard,Bertrand,Canet,Thery,Remuson,Jeminet
, p. 2431 - 2434 (2000)
Enantiomeric analysis and empirical dete...
Enantioselective Cascade Biocatalysis for Deracemization of Racemic β-Amino Alcohols to Enantiopure (S)-β-Amino Alcohols by Employing Cyclohexylamine Oxidase and ω-Transaminase
Zhang, Jian-Dong,Chang, Ya-Wen,Dong, Rui,Yang, Xiao-Xiao,Gao, Li-Li,Li, Jing,Huang, Shuang-Ping,Guo, Xing-Mei,Zhang, Chao-Feng,Chang, Hong-Hong
, p. 124 - 128 (2020/09/21)
Optically active β-amino alcohols are ve...
Highly Stable Zr(IV)-Based Metal-Organic Frameworks for Chiral Separation in Reversed-Phase Liquid Chromatography
Jiang, Hong,Yang, Kuiwei,Zhao, Xiangxiang,Zhang, Wenqiang,Liu, Yan,Jiang, Jianwen,Cui, Yong
supporting information, p. 390 - 398 (2021/01/13)
Separation of racemic mixtures is of gre...
Rational Design of Chiral Nanohelices from Self-Assembly of Meso-tetrakis (4-Carboxyphenyl) Porphyrin-Amino Acid Conjugates
Yang, Xuejiao,Shen, Yuhe,Liu, Jiayu,Wang, Yuefei,Qi, Wei,Su, Rongxin,He, Zhimin
, p. 13067 - 13074 (2021/11/16)
In this article, meso-tetrakis (4-carbox...
Generation of Oxidoreductases with Dual Alcohol Dehydrogenase and Amine Dehydrogenase Activity
Tseliou, Vasilis,Schilder, Don,Masman, Marcelo F.,Knaus, Tanja,Mutti, Francesco G.
supporting information, p. 3315 - 3325 (2020/12/11)
The l-lysine-?-dehydrogenase (LysEDH) fr...
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego