Cosmetics Grade Telaprevir 402957-28-2 For Sale with Good Price
- Molecular Formula:C36H53N7O6
- Molecular Weight:679.86
- Appearance/Colour:White powder
- PKA:11.84±0.20(Predicted)
- PSA:179.56000
- Density:1.25 g/cm3
- LogP:3.94740
Telaprevir(Cas 402957-28-2) Usage
|
Description
|
Telaprevir is a drug developed by Vertex Pharmaceuticals and Johnson & Johnson under the brand names Incivek and Incivo for the treatment of hepatitis C. It is a member of a class of antiviral drugs called protease inhibitors. |
|
Uses
|
Telaprevir works by decreasing the amount of hepatitis C virus (HCV) in the body. Telaprevir may not prevent the spread of hepatitis C to other people. Telaprevir is a NS3/4a protease inhibitor used to inhibit viral HCV replication. NS3/4a protease is an integral part of viral replication and mediates the cleavage the virally encoded polyprotein to mature proteins (NS4A, NS4B, NS5A and NS5B) Label. Telaprevir inhibits NS3/4A with an IC50 of 10nM. |
|
Metabolism
|
Extensively metabolised in the liver, involving hydrolysis, oxidation, and reduction. Multiple metabolites were detected in faeces, plasma and urine.
|
|
Definition
|
ChEBI: An oligopeptide consisting of N-(pyrazin-2-ylcarbonyl)cyclohexylalanyl, 3-methylvalyl, octahydrocyclopenta[c]pyrrole-1-carboxy, and 3-amino-N-cyclopropyl-2-oxohexanamide residues joined in sequence. Used f r treatment of chronic hepatitis C virus genotype 1 infection.
|
|
Brand name
|
Incivek
|
InChI:InChI=1/C36H53N7O6/c1-5-10-25(29(44)34(48)39-23-15-16-23)40-33(47)28-24-14-9-13-22(24)20-43(28)35(49)30(36(2,3)4)42-32(46)27(21-11-7-6-8-12-21)41-31(45)26-19-37-17-18-38-26/h17-19,21-25,27-28,30H,5-16,20H2,1-4H3,(H,39,48)(H,40,47)(H,41,45)(H,42,46)/t22-,24-,25?,27-,28-,30+/m0/s1
402957-28-2 Relevant articles
Review of Boceprevir and Telaprevir for the Treatment of Chronic Hepatitis C
Kyle J Wilby, Nilufar Partovi, Jo-Ann E Ford, Erica D Greanya, Eric M Yoshida
, Canadian Journal of Gastroenterology and Hepatology, 2012
The studies identified assessed boceprevir and telaprevir in genotype-1 hepatitis C patients. In both treatment-naive and treatment-experienced patients, sustained virological response rates were achieved more often with boceprevir or telaprevir in combination with pegylated interferon and ribavirin compared with pegylated interferon and ribavirin alone. Both medications were well tolerated, with anemia presenting as the most treatment-limiting adverse effect.
Telaprevir with Peginterferon and Ribavirin for Chronic HCV Genotype 1 Infection
John G. McHutchison, M.D., Gregory T. Everson, M.D., Stuart C. Gordon, M.D., Ira M. Jacobson, M.D., Mark Sulkowski, M.D., Robert Kauffman, M.D., Lindsay McNair, M.D., John Alam, M.D., and Andrew J. Muir, M.D., for the PROVE1 Study Team*
, N Engl J Med 2009;360:1827-1838
Viral breakthrough occurred in 7% of patients receiving telaprevir. The rate of discontinuation because of adverse events was higher in the three telaprevir-based groups (21%, vs. 11% in the PR48 group), with rash the most common reason for discontinuation. Treatment with a telaprevir-based regimen significantly improved sustained virologic response rates in patients with genotype 1 HCV, albeit with higher rates of discontinuation because of adverse events.
Response-Guided Telaprevir Combination Treatment for Hepatitis C Virus Infection
Kenneth E. Sherman, M.D., Ph.D., Steven L. Flamm, M.D., Nezam H. Afdhal, M.D., David R. Nelson, M.D., Mark S. Sulkowski, M.D., Gregory T. Everson, M.D., Michael W. Fried, M.D.
, N Engl J Med 2011;365:1014-1024
We enrolled patients with chronic infection with HCV genotype 1 who had not previously received treatment. All patients received telaprevir at a dose of 750 mg every 8 hours, peginterferon alfa-2a at a dose of 180 μg per week, and ribavirin at a dose of 1000 to 1200 mg per day, for 12 weeks (T12PR12), followed by peginterferon–ribavirin.
402957-28-2 Process route
-
- 402957-28-2
(1S,3aR,6aS)-2-((S)-2-((S)-2-cyclohexyl-2-(pyrazine-2-carboxamido)acetamido)-3,3-dimethylbutanoyl)-N-((S)-1-(cyclopropylamino)-1,2-dioxohexan-3-yl)octahydrocyclopenta[c]pyrrole-1-carboxamide
Conditions
| Conditions |
Yield |
|
C38H57N7O7; With methanol; potassium carbonate; at 20 ℃; for 0.5h;
With Dess-Martin periodane; In dichloromethane; at 20 ℃;
|
80% |
|
C38H57N7O7; With potassium carbonate; In methanol;
With Dess-Martin periodane; In dichloromethane;
|
80% |
-
- 402959-36-8
(1S,3aR,6aS)-2-[(2S)-2-[[(2S)-2-cyclohexyl-2-[(2-pyrazinylcarbonyl)amino] acetyl]amino]-3,3-dimethylbutanoyl]-N-[(1S)-1-[(cyclopropylamino)(hydroxy)acetyl]butyl]-3,3a,4,5,6,6a-hexahydro-1H-cyclopenta[c]pyrrole-3-carboxamide
-
- 402957-28-2
(1S,3aR,6aS)-2-((S)-2-((S)-2-cyclohexyl-2-(pyrazine-2-carboxamido)acetamido)-3,3-dimethylbutanoyl)-N-((S)-1-(cyclopropylamino)-1,2-dioxohexan-3-yl)octahydrocyclopenta[c]pyrrole-1-carboxamide
Conditions
| Conditions |
Yield |
|
With Dess-Martin periodane; In dichloromethane; at 0 - 5 ℃; for 2h;
|
93.61% |
|
With sodium hypochlorite; sodium hydrogencarbonate; sodium bromide; In dichloromethane; water; at 0 - 5 ℃;
|
90% |
|
With sodium hypochlorite; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; sodium hydrogencarbonate; In dichloromethane; water; at 0 - 25 ℃; for 3 - 4h; Product distribution / selectivity;
|
85% |
|
With sodium hypochlorite; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; sodium hydrogencarbonate; potassium bromide; In dichloromethane; at 20 ℃; for 20h; Cooling with ice;
|
71% |
|
With Dess-Martin periodane; In dichloromethane; at 0 - 5 ℃; for 2h;
|
66% |
|
With sodium hypochlorite; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; sodium hydrogencarbonate; sodium bromide; In dichloromethane; water; at 5 - 8 ℃; for 1h; Product distribution / selectivity;
|
|
|
With Dess-Martin periodane; In dichloromethane; at 20 ℃;
|
0.412 g |
402957-28-2 Upstream products
-
402959-36-8
(1S,3aR,6aS)-2-[(2S)-2-[[(2S)-2-cyclohexyl-2-[(2-pyrazinylcarbonyl)amino] acetyl]amino]-3,3-dimethylbutanoyl]-N-[(1S)-1-[(cyclopropylamino)(hydroxy)acetyl]butyl]-3,3a,4,5,6,6a-hexahydro-1H-cyclopenta[c]pyrrole-3-carboxamide
-
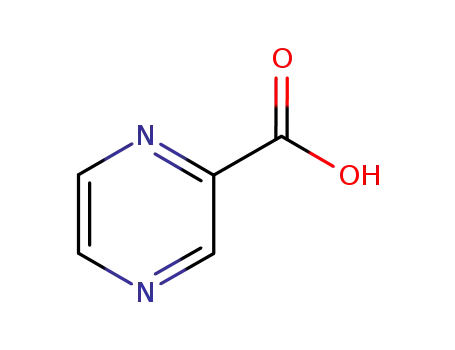
98-97-5
2-pyrazylcarboxylic acid
-
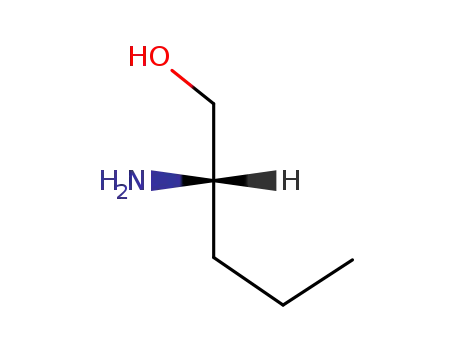
22724-81-8
L-norvalinol
-
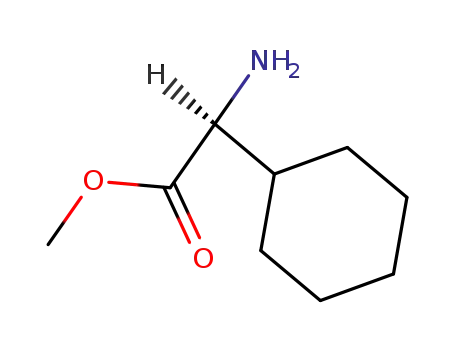
145618-11-7
(S)-amino(cyclohexyl)acetic acid methyl ester
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego




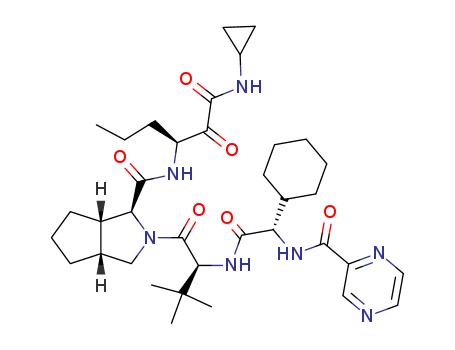

![(1S,3aR,6aS)-2-((S)-2-((S)-2-cyclohexyl-2-(pyrazine-2-carboxamido)acetamido)-3,3-dimethylbutanoyl)-N-((S)-1-(cyclopropylamino)-1,2-dioxohexan-3-yl)octahydrocyclopenta[c]pyrrole-1-carboxamide](/upload/2024/8/fa38f662-22bb-41cb-84c3-79ce8ad8f944.png)
![(1S,3aR,6aS)-2-[(2S)-2-[[(2S)-2-cyclohexyl-2-[(2-pyrazinylcarbonyl)amino] acetyl]amino]-3,3-dimethylbutanoyl]-N-[(1S)-1-[(cyclopropylamino)(hydroxy)acetyl]butyl]-3,3a,4,5,6,6a-hexahydro-1H-cyclopenta[c]pyrrole-3-carboxamide](/upload/2024/8/9eed6bf6-ee64-41d7-be56-d006950fcc2a.png)