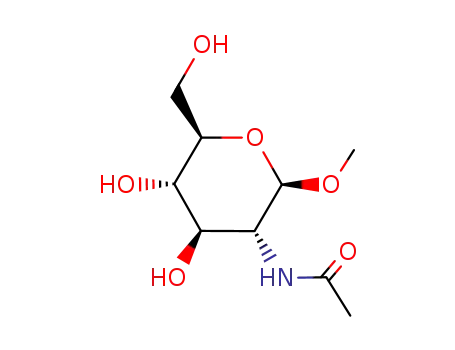
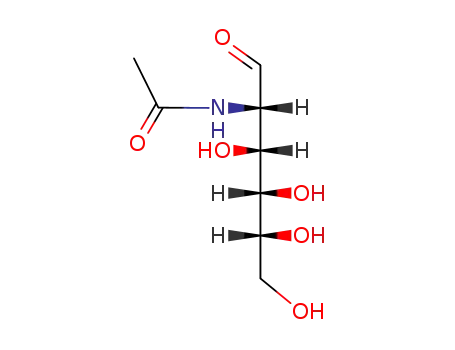
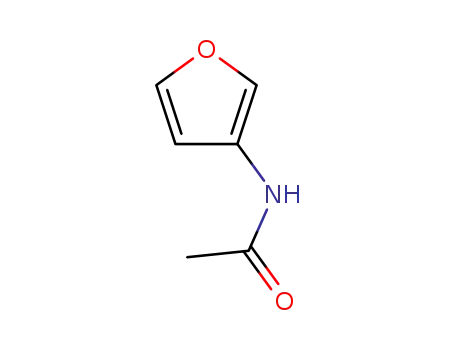
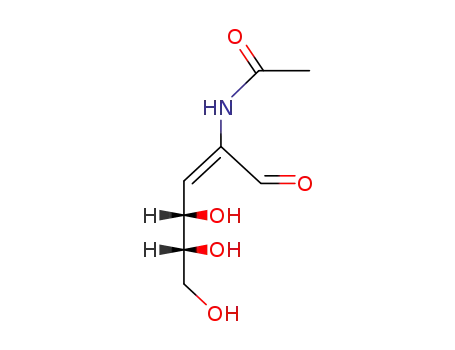
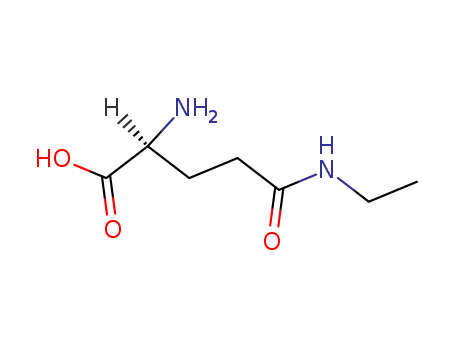
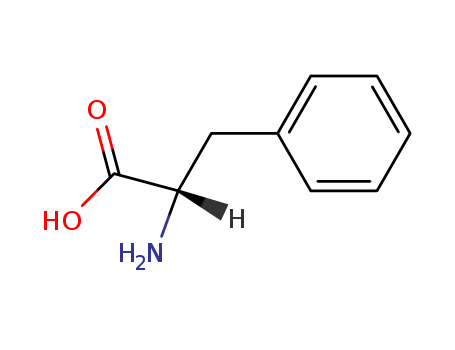
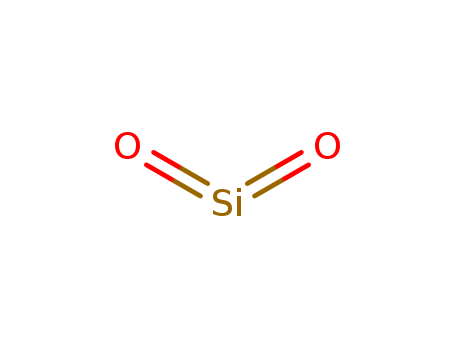
Yeast-mycelial conversion induced by N-acetyl-D-glucosamine in Candida albicans
N. SIMONETTI, V. STRIPPOLI & A. CASSONE
, Nature volume 250, pages344–346 (1974)
DIMORPHISM in fungi is generally defined as a reversible transition from a yeast habit of growth (Y) to a mycelial one (M)1. In Candida albicans Y→M transition can occur rapidly in serum, serum substitutes and other natural4–6 and synthetic media. In a few hours the yeast cell or blastospore forms a germ tube which grows as a true mycelium.
Anti-tumor properties of orally administered glucosamine and N-acetyl-d-glucosamine oligomers in a mouse model
Sachie Masuda a , Kazuo Azuma a 1 , Seiji Kurozumi a , Masatoshi Kiyose a , Tomohiro Osaki a , Takeshi Tsuka a , Norihiko Itoh a , Tomohiro Imagawa a , Saburo Minami a , Kimihiko Sato b , Yoshiharu Okamoto a
, Carbohydrate Polymers Volume 111 , 13 October 2014, Pages 783-787
Previous reports have indicated that N-acetyl-d-glucosamine oligomer (NACOS) and glucosamine oligomer (COS) possess anti-tumor properties (Harish-Prashanth and Tharanathan, 2005, Huang et al., 2006, Shen et al., 2009; Suzuki et al., 1986, Tokoro et al., 1989, Wang et al., 2008). Furthermore, it was revealed that the tumor inhibitory effects of NACOS and COS are potentially related to their ability to induce lymphocyte cytokines thorough increased T-cell proliferation.
Biochemical Characterization and Structural Analysis of a β- N-Acetylglucosaminidase from Paenibacillus barengoltzii for Efficient Production of N-Acetyl- d -glucosamine
Liu, Yihao,Jiang, Zhengqiang,Ma, Junwen,Ma, Shuai,Yan, Qiaojuan,Yang, Shaoqing
, p. 5648 - 5657 (2020/06/03)
Bioproduction of N-acetyl-d-glucosamine ...
Synthesis and anticholinesterase activities of novel glycosyl benzoxazole derivatives
Cao, Zhi-Ling,Liu, Shu-Hao,Liu, Wei-Wei,Ren, Shu-Ting,Shi, Da-Hua,Wang, Lei,Wang, You-Xian,Wu, Yu-Ran
, p. 363 - 366 (2020/02/05)
Eight glycosyl benzoxazole derivatives a...
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego




![3-O-{α-D-xylopyranosyl-(1→3)-α-L-arabinopyranosyl-(1→6)-[β-D-glucopyranosyl-(1→3)]-2-(acetamido)-2-deoxy-β-D-glucopyranosyl}echinocystic acid 28-O-{β-D-apiofuranosyl-(1→3)-β-D-xylopyranosyl-(1→2)-[2-O-cinnamoyl-α-L-arabinopyranosyl-(1→4)]-6-O-acetyl-β-D-glucopyranosyl} ester](/upload/2024/8/5d9e4b26-6adb-492c-9658-f025560c7f2e.png)


![3-O-{α-D-xylopyranosyl-(1→3)-α-L-arabinopyranosyl-(1→6)-[β-D-glucopyranosyl-(1→3)]-2-(acetamido)-2-deoxy-β-D-glucopyranosyl}echinocystic acid 28-O-(β-D-apiofuranosyl-(1→3)-β-D-xylopyranosyl-(1→2)-{2-O-[(6S,2E)-2,6-dimethyl-6-hydroxy-2,7-octadienoyl]-α-L-arabinopyranosyl-(1→4)}-6-O-acetyl-β-D-glucopyranosyl) ester](/upload/2024/8/cd51bdca-bfa4-42f4-a2b0-f592aeaffaa1.png)