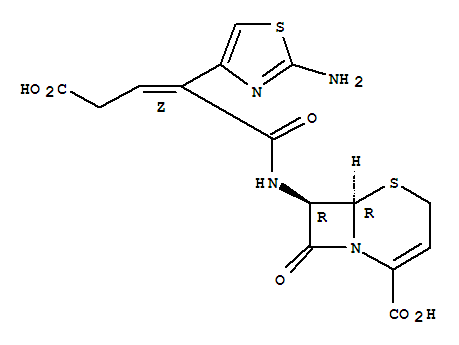
Cosmetics Grade Ceftibuten 97519-39-6 For Sale with Good Price
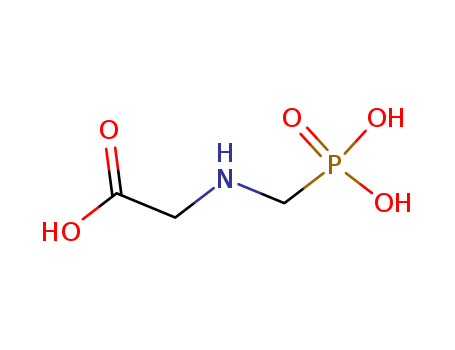
- Molecular Formula:C15H14N4O6S2
- Molecular Weight:410.42
- Vapor Pressure:0mmHg at 25°C
- Refractive Index:1.762
- Boiling Point:966.4 °C at 760 mmHg
- PKA:2.99±0.50(Predicted)
- Flash Point:538.3 °C
- PSA:216.46000
- Density:1.75 g/cm3
- LogP:0.86180
Ceftibuten(Cas 97519-39-6) Usage
| Description |
Ceftibuten is a third-generation cephalosporin antibiotic used to treat bacterial infections, including bronchitis and infections of the ears, throat, tonsils, and urinary tract. It works by inhibiting bacterial cell wall synthesis, effectively killing bacteria. Ceftibuten is highly effective against many Gram-negative bacilli but has poor activity against Gram-positive cocci. It is stable against plasmid-mediated β-lactamases but not derepressed chromosomal enzymes. Orally administered, ceftibuten is rapidly absorbed, with a bioavailability of 75-90%, though this is reduced when taken with food. It has a plasma protein binding of 65-77% and a half-life of 1.5-3 hours. Despite poor in vitro activity against Streptococcus pneumoniae, clinical trials show its efficacy in treating respiratory and urinary tract infections. Side effects are generally mild, including gastrointestinal symptoms and changes in liver function tests. Ceftibuten was sold under brand names like Cedax and Seftem. |
|
Manufacturing Process
|
The 1st method of synthesisThe 8-oxo-7-phenylacetylamino-5-thia-1-aza-bicyclo[4.2.0]oct-1-ene-2- carboxylic acid benzhydryl ester is reacted with phosphorus pentachloride/pyridine reagent in methylene dichloride, and the reaction mixture is thereafter cooled to -35°C and treated with methanol to produce hydrochloride of 7-amino-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2- carboxylic acid benzhydryl ester. This hydrochloride is reacted with 4-(3- aminothiophen-2-yl)-5-oxohex-3-enoic acid 3-methylbut-2-enyl ester. Then 7- [2-(2-benzoylamino-thiazol-5-yl)(3-tert-butyl-4,4-dimethylpent-2- enoxycarbonyl)-pent-2-enoylamino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2- ene-2-carboxylic acid synthesized is reacted with aluminum chloride in anisole and diluted hydrochloric acid and then with dimethylmalonate to give 5-thia- 1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid, 7-(((2Z)-2-(2-amino-4- thiazolyl)-4-carboxy-1-oxo-2-butenyl)amino)-8-oxo-, (6R,7R)- (Ceftibuten).The 2st method of synthesisFormulation of the diphenylmethyl thiazoleacetate with ethyl formate leads to 2-(2-aminothiazol-5-yl)-3-hydroxyacrylic acid benzhydryl ester. Condensation of 2-(2-aminothiazol-5-yl)-3-hydroxyacrylic acid benzhydryl ester with the phosphorane from benzyl 2-triphenylphosphonium acetate leads to the 2-(2- aminothiazol-5-ylmethylene)succinic acid 1-benzhydryl ester 4-benzyl ester. Exposure of this ester to trifluoroacetic acid selectively cleaves the diphenylmethyl group over the benzyl ester to give 2-(2-aminothiazol-5- ylmethylene)succinic acid 4-benzyl ester. Condensation of the acid with free amino group in the desmethyl cephalosporin affords the amide of 7-[3-(2- aminothiazol-5-yl)-2-benzoylcarbonylmethylacryloylamino)-8-oxo-5-thia-1- azabicyclo[4.2.1]oct-2-ene-2-carboxylic acid benzyl ester. The remaining benzyl ester protecting groups are removed by means of aluminum chloride to afford 7-[3-(2-aminothiazol-5-yl)-2-benzoylcarbonylmethylacryloylamino)-8- oxo-5-thia-1-azabicyclo[4.2.1]oct-2-ene-2-carboxylic acid or ceftibuten. Shanghai Wibson Biotechnology Co., Ltd. is a high-tech enterprise that mainly develops and sells pharmaceutical and chemical raw materials and other related products. The company has advanced and perfect supporting system and has formed advanced chemical synthesis technology, which can quickly meet the personalized needs and series development from suitable testing to industrial production. Products are exported to Russia, Southeast Asia and other countries, in the world has a very high reputation and visibility. The company has strong financial strength and a good operating environment, can provide customers with high-quality, efficient and fast services.
|
|
Biochem/physiol Actions
|
Ceftibuten is a third generation cephalosporin antibiotic
|
|
Pharmacokinetics
|
Ceftibuten is highly (75–90%) absorbed on oral administration, but this is decreased significantly by food. Being lipophilic and acidic, it is significantly (65%) serum protein bound. Some isomerization of the geometry of the olefinic linkage appears to take place in vivo before excretion.
|
|
Definition
|
ChEBI: A third-generation cephalosporin antibiotic with a [(2Z)-2-(2-amino-1,3-thiazol-4-yl)-4-carboxybut-2-enoyl]amino substituent at the 7 position of the cephem skeleton. An orally-administered agent, ceftibuten is used as the dihydrate to tre t urinary-tract and respiratory-tract infections.
|
|
Brand name
|
Cedax (Schering);Seftem.
|
| Referances |
Ceftibuten: A Review of its Antibacterial Activity, Pharmacokinetic Properties and Clinical Efficacy
, Lynda R. Wiseman & Julia A. Balfour
, Drugs, Volume 47, pages 784–808, (1994)
b-Haemolytic streptococci groups A (Streptococci pyogenes), C, F and G were highly susceptible to ceftibuten, whereas Group B (S. agalactiae) were generally resistant. Ceftibuten inhibited penicillin-susceptible strains of S. pneumoniae but was inactive against penicillin-resistant strains, and was also inactive against staphylococci, enterococci and Listeria spp. Ceftibuten showed high affinity for penicillin binding protein (PBP) 3 of E. coli and PBPs 2, 4 and 5 of H. influenzae. Minimum bactericidal concentrations of ceftibuten against most susceptible bacteria were generally similar to, or within 4-fold, of minimum inhibitory concentrations (MIC), and ceftibuten showed a postantibiotic effect ranging from 1 to > 10 hours against the respiratory pathogens S. pneumoniae, S. pyogenes, H. influenzae and M. catarrhalis.
|
InChI:InChI=1/C15H14N4O6S2/c16-15-17-7(5-27-15)6(1-2-9(20)21)11(22)18-10-12(23)19-8(14(24)25)3-4-26-13(10)19/h1,3,5,10,13H,2,4H2,(H2,16,17)(H,18,22)(H,20,21)(H,24,25)/b6-1+
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego