|
Chemical Composition
|
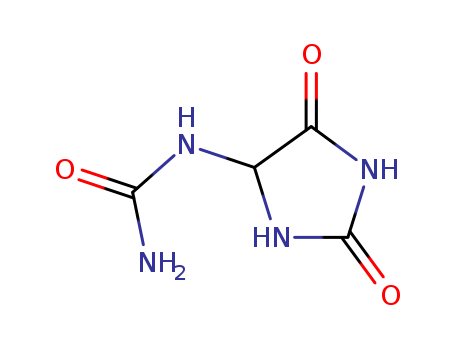
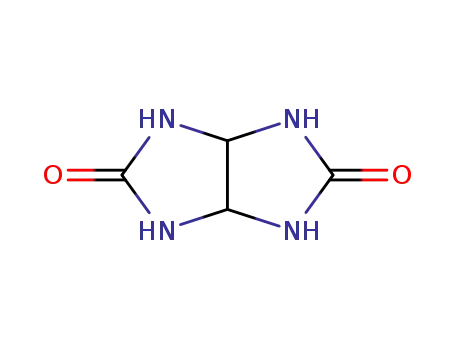
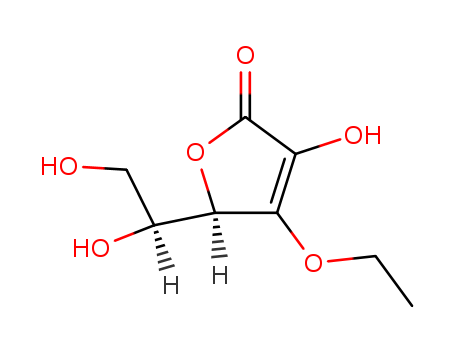
Allantoin, chemically known as (2,5-dioxo-4-imidazolidinyl)urea, consists of a heterocyclic compound with a five-membered ring containing a urea substituent in the 4th position.
|
|
Biological Properties
|
Acts as an intermediate product of the purine catabolic pathway in plants, aiding in nitrogen mobilization.
Plays a role in increasing stress tolerance in plants, including drought, salt, cold, heavy metals, and irradiance.
Enhances wound healing process by modulating the inflammatory response, stimulating fibroblast proliferation, and extracellular matrix synthesis.
Facilitates inter-plant interactions and kin recognition in plants, acting as a signaling molecule.
|
|
Applications in Skin Care
|
Widely used in skin care products due to its ability to promote healing of scar tissue and scars.
Found in over 1300 cosmetic products. Originally discovered in the callus of the Coffea Arabica plant.
|
|
Synthetic Potential
|
Allantoin's availability and multifunctionality make it attractive for synthetic purposes, especially in the pharmaceutical industry. Its chemical properties include hydrolysis reactions, complexation with organic and inorganic substrates, and interactions with nucleophilic reagents.
Can serve as a basic azaheterocycle to manufacture a wide range of new compounds, including bioactive ones.
|
|
Anti-inflammatory analgesic effects
|
Allantoin has anti-inflammatory analgesic effects, but it also has a weak partial paralysis effect, can effectively reduce stimuli of stimulus, can be used as a skin protectant and anti-irritant, can reduce skin irritation of cosmetic ingredients, China food and Drug Administration rank it as the first kind efficient active ingredient of skin care agent, now has been widely used in many products such as shampoo, sunscreen products, creams and lotions, shaving creams and oral care products.
|
|
Chemical properties
|
Colorless crystalline powder. Melting point is 238-240 ℃ (decomposition). Can be dissolved in hot water, hot alcohol and dilute sodium hydroxide solution, slightly soluble in water and alcohol, almost insoluble in ether and chloroform. Odorless, tasteless. In dry air is stable, prolonged boiling in the water. or strong base will be destroyed. pH of saturated aqueous solution is 5.5. The above information is edited by the lookchem of Liu Yujie.
|
|
Definition
|
ChEBI: An imidazolidine-2,4-dione that is 5-aminohydantoin in which a carbamoyl group is attached to the exocyclic nitrogen.
|
|
Manufacturing Process
|
To a mixture of 13.14 kg 40% solution of glyoxal in water and a solution of 0.5 L of concentrated HCl in 3 L of water was added 1.8 g Co(NO3)2·6H2O. The mixture was heated at 50-60°C, to the solution was added 20 g of sodium nitrite and then at 40-60°C was added dropwise the mixture of 6 L of concentrated HNO3, 4.2 L of water and 30 g of sodium nitrite. The product obtained was mixed with 2.4 kg ammonium sulfate and filtrated. The filtrate was heated with 14.5 kg urea at 70°C for 10 hours. Allantoin was filtrated and recrystallized from the water; M.P. 233-235°C.
|
|
Therapeutic Function
|
Vulnerary, Antiulcer (topical), Antipsoriatic
|
|
General Description
|
Allantoin, the final catabolic product of purines in mammals, is a highly polar and amphoteric substance. Allantoin is mainly used for skin protection as the drug promotes cell proliferation and wound healing.Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
|
|
Flammability and Explosibility
|
Nonflammable
|
|
Biochem/physiol Actions
|
Purine metabolite via the uric acid pathway. Uric acid also reacts with free radicals to produce allantoin, thus allantoin may be a useful biomarker for oxidative stress.
|
|
Purification Methods
|
It crystallises from water or EtOH [Hartman et al. Org Synth Coll Vol II 21 1943]. [Beilstein 25 III/IV 4071.]
|
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego