|
Description
|
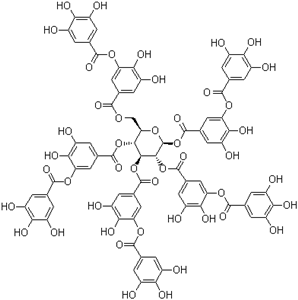
Tannic acid is a naturally occurring polyphenol found in plants, fruits, and seeds. It is classified as a type of hydrolyzable tannin, formed through the esterification of gallic acid and glucose. |
| Uses |
Leather Tanning: Widely used in the leather industry to tan hides and skins, improving their durability and texture.
Textile Dyeing: Serves as a mordant (fixative) for dyes in cotton and other fabrics.
Ink Manufacturing: A key ingredient in the production of inks, especially historical iron gall ink. |
|
Production Methods
|
Extracted from nutgalls, twigs of certain oak species, or the seed pods of Caesalpinia spinosa.
Typically involves solvent extraction followed by concentration, and in some cases, chemical synthesis. |
|
Physical and Chemical Properties
|
Physical Properties:
Pale yellow amorphous powder or solid.
Soluble in water, ethanol, and methanol.
Weakly acidic in aqueous solutions.
Darkens on exposure to air and light.
Chemical Properties:
Reacts with trivalent iron ions, producing a blue-black color.
Forms precipitates with gelatin, alkaloids, and heavy metal ions.
Decomposes at 210°C, yielding pyrogallic acid and carbon dioxide.
Exhibits antioxidant, antimutagenic, and weak carcinogenic properties. |
| Toxicity |
Poisonous if ingested or inhaled in large quantities.
Can cause irritation to the respiratory tract, gastrointestinal issues (vomiting, diarrhea), and potential liver damage.
Questionable carcinogen based on experimental data. |
| Biodegradability |
Naturally occurring in plants, tannic acid degrades in the environment, especially through microbial action. |
|
Reference quality standards
|
In 1985 the US Food and Drug Administration had listed tannic acid as “general recognized as safe” (GRAS), meaning that it is not harmful or dangerous. It can be used in pharmaceutical, cosmetic preparation; as the ingredients as food such as frozen dairy products, meat products or drinks such as soft drinks, tea, wine ingredients. Figure 3 is the reference quality standards of industry tannic acid and pharmaceutical tannic acid
|
|
Category
|
toxic substances
|
|
Toxicity grading
|
highly toxic
|
|
Acute toxicity
|
intraperitoneal-mouse LD50: 150 mg/kg; intravenous-mouse LD50: 50 mg/kg
|
|
Flammability and hazard properties
|
thermal decomposition yields acrid smoke
|
|
Storage characteristics
|
warehouse: low-temperature, dry and ventilated
|
|
Extinguishing media
|
Water, carbon dioxide, foam, powder
|
|
Preparation
|
Tannic acid is obtained by solvent extraction from the nutgalls or the excrescences that form on the young twigs of Quercus olivier and allied species of Quercus L.; from the seed pods of Tara (Caesalpinia spinosa); or from the nutgalls of various sumac species, including Rhus semialata, R. coriaria, R. glabra and R. typhia.
|
|
Composition
|
Main constituents include three crystalline alkaloids—aspidospermine, quebrachine and quebrachamine (chemically identical to yohimbine)—responsible for the tonic and antispasmodic physiological action. A. quebrachoblanco bark contains 0.3 to 1.5% alkaloids (aspidospermine 30%, quebrachine 10%, deacetylaspidospermine 5%, aspidospermatidine 3%, 1-methylaspidospermatidine 0.5%, quebrachimine and quebrachit). Leaves contain rhazinilam (lactam).
|
|
Air & Water Reactions
|
Water soluble. Gradually darkens on exposure to air and/or light
|
|
Reactivity Profile
|
Glycerite decomposes at 210° to carbon dioxide and pyrogallol, which can form irritating vapors [USCG, 1999]. Incompatible with salts of heavy metals, alkaloids, gelatin, albumin, starch, oxidizing substances-e.g., permanganates, chlorates; lime water [Merck].
|
|
Safety Profile
|
Poison by intravenous and intraperitoneal routes. Questionable carcinogen with experimental tumorigenic data. When heated to decomposition it emits acrid smoke and irritating fumes.
|
|
Environmental Fate
|
Tannins and tannic acid occur naturally in plants. Essentially all wood and plant tissue contain tannins. Therefore, biodegradation is expected to be the major environmental fate process for tannic acid.
|
|
Toxicity evaluation
|
Tannic acid causes centrilobular liver necrosis following absorption from gastrointestinal tract, mucus membranes, or denuded skin surfaces. Liver metabolism of tannic acid requires methyl-group donors. Therefore, methyl-group donors can be depleted following excessive tannic acid absorption.
|
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego