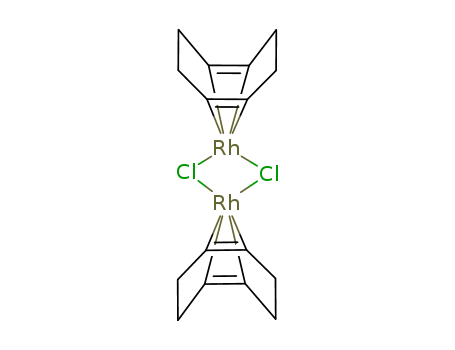
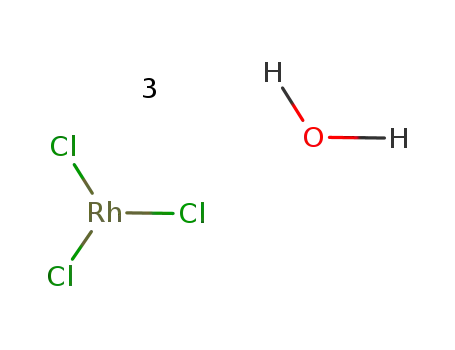
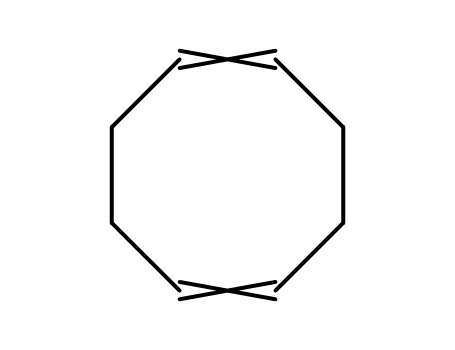
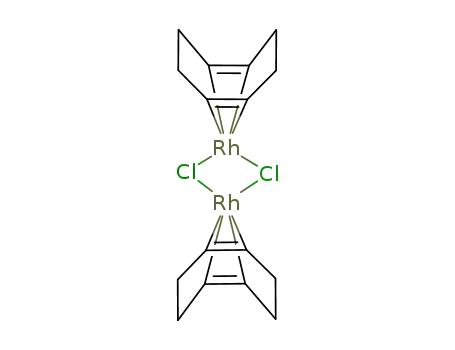
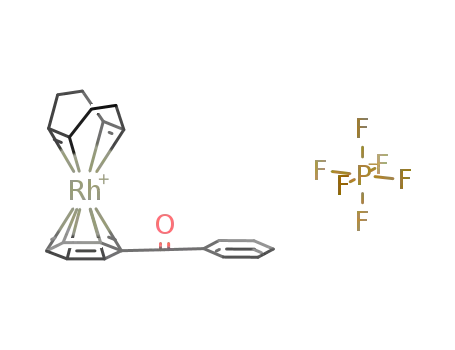Export Top Purity Chloro(1,5-cyclooctadiene)rhodium(I) dimer 12092-47-6 In Stock
- Molecular Formula:C16H24Cl2Rh2
- Molecular Weight:493.084
- Appearance/Colour:orange crystals
- Vapor Pressure:0Pa at 25℃
- Melting Point:243 °C (dec.)(lit.)
- Boiling Point:153.5 °C at 760 mmHg
- Flash Point:31.7 °C
- PSA:0.00000
- Density:1.94[at 20℃]
- LogP:-0.64640
Chloro(1,5-cyclooctadiene)rhodium(I) dimer(Cas 12092-47-6) Usage
| Description |
Chloro(1,5-cyclooctadiene)rhodium(I) dimer is primarily classified as an organometallic compound due to its rhodium-metal center and organic ligands. It is used widely as a precursor to homogeneous catalysts in various organic and inorganic chemical reactions. Chloro(1,5-cyclooctadiene)rhodium(I) dimer is an organometallic complex that consists of two rhodium atoms in the +1 oxidation state. Each rhodium atom is bonded to a chloride ion and a 1,5-cyclooctadiene (COD) ligand. The two rhodium atoms are bridged by chloride ions, forming a dimeric structure. This complex is highly valued for its role as a catalyst precursor in many chemical transformations, particularly in homogeneous catalysis. |
| Structure |
Dimeric complex with two rhodium centers bridged by chloride ions. The rhodium atoms are coordinated with 1,5-cyclooctadiene (COD) ligands. |
| Solubility |
Soluble in common organic solvents such as benzene, dichloromethane, and toluene. |
| Stability |
Stable under ambient conditions but may decompose upon exposure to moisture or light over time. |
| Uses |
Widely used in homogeneous catalysis, including reactions like hydrogenation, hydroformylation, and carbon-carbon bond formation. Chloro(1,5-cyclooctadiene)rhodium(I) dimer acts as a precursor for generating active rhodium species in situ, which can catalyze a variety of transformations. |
InChI:InChI=1/2C8H12.2ClH.2Rh/c2*1-2-4-6-8-7-5-3-1;;;;/h2*1-2,7-8H,3-6H2;2*1H;;/q;;;;2*+1/p-2/b2*2-1-,8-7-;;;;/r2C8H12.2ClRh/c2*1-2-4-6-8-7-5-3-1;2*1-2/h2*1-2,7-8H,3-6H2;;/b2*2-1-,8-7-;;
12092-47-6 Relevant articles
Application of microwave dielectric loss heating effects for the rapid and convenient synthesis of organometallic compounds
Baghurst, David R.,Mingos, D. Michael P.,Watson, Michael J.
, p. C43 - C45 (1989)
Diolefin-rhodium(I) and -iridium(I) comp...
Design and application of a reflux modification for the synthesis of organometallic compounds using microwave dielectric loss heating effects
Baghurst, David R.,Mingos, D. Michael P.
, p. C57 - C60 (1990)
A commercially available microwave oven ...
Immobilized chiral rhodium nanoparticles stabilized by chiral P-ligands as efficient catalysts for the enantioselective hydrogenation of 1-phenyl-1,2-propanedione
Ruiz, Doris,M?ki-Arvela, P?ivi,Aho, Atte,Chiment?o, Ricardo,Claver, Carmen,Godard, Cyril,Fierro, José L.G.,Murzin, Dmitry Yu.
, (2019)
This work reports the efficient synthesi...
Synthesis of M2Rh2 Bis(μ3-carbon dioxide) complexes from the reaction between [Rh(OH) (η4-COD)]2 and cationic metal carbonyls
Tetrick, Stephen M.,Xu, Chongfu,Pinkes, John R.,Cutler, Alan R.
, p. 1861 - 1867 (1998)
The M2Rh2 bis(μ3-CO2) complexes [Cp*(CO)...
Reactions of [RhCl(diene)]2 with Bi- and terdentate nitrogen ligands. X-ray structures of five-coordinate complexes
Haarman, Hendrikus F.,Bregman, Frank R.,Ernsting, Jan-Meine,Veldman, Nora,Spek, Anthony L.,Vrieze, Kees
, p. 54 - 67 (2008/10/08)
Reaction of [RhCl(diene)]2 (diene = 1,5-...
12092-47-6 Process route
-
-
rhodium(III) chloride trihydrate
-
- 5259-72-3,10060-40-9,111-78-4
cyclo-octa-1,5-diene
-
- 12092-47-6
di-μ-chloro-bis(1,5-cyclooctadiene)dirhodium
Conditions
| Conditions |
Yield |
|
In ethanol; water; in a microwave oven (Fischer-Porter reaction vessel) at 140°C for 0.5 min; elem. anal.;
|
84% |
|
In ethanol; water; under N2 or Ar, reflux of soln. overnight; soln. is cooled, recrystn. from CH2Cl2/hexane;
|
82% |
|
In ethanol; A soln. of RhCl*3H2O and COD is refluxed for 45 min until a ppt. sepd. out.; Ppt. is washed with CH3OH, dried at 80°C for 30 min, recrystd. from petroleum ether (60-80°C).;
|
|
-
-
rhodium trichloride hydrate
-
- 12092-47-6
di-μ-chloro-bis(1,5-cyclooctadiene)dirhodium
Conditions
| Conditions |
Yield |
|
With cyclooctadiene; In ethanol; water; in a modified reflux microwave apparatus RhCl3 reacts with cyclooctadiene in EtOH-H2O (5:1) (react. time: 25 min);
|
87% |
12092-47-6 Upstream products
-
5259-72-3
cyclo-octa-1,5-diene
-
80319-87-5
C6H5CH(C3H(CH3)2N2)2Rh(C8H12)(1+)*Cl(1-)
-
102533-99-3
RhCl(Hpz)(η4-cyclooca-1,5-diene)
-
129370-99-6
chloro(η4-1,5-cyclooctadiene)(1-oxa-4-telluracyclohexane)rhodium(I)
12092-47-6 Downstream products
-
101307-69-1
{Rh(C8H12)(η6-(C6H5)2CO)}PF6
-
82861-87-8
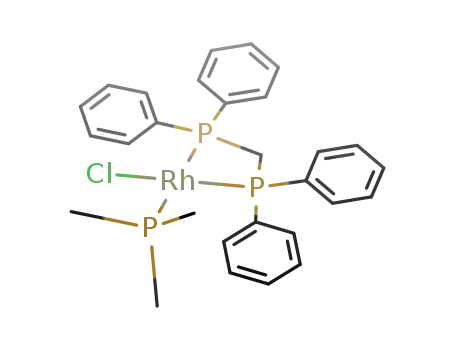
RhCl(trimethylphosphine)(bis(diphenylphosphino)methane)
-
136695-92-6
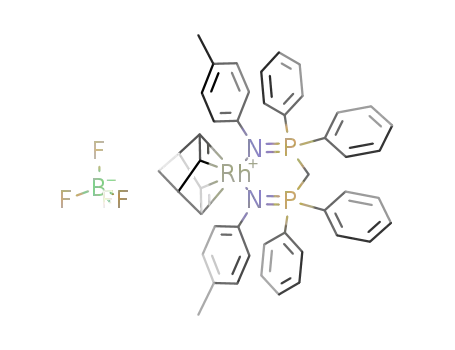
{Rh(norbornadiene)((4-CH3C6H4N=PPh2)2CH2)}BF4
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego