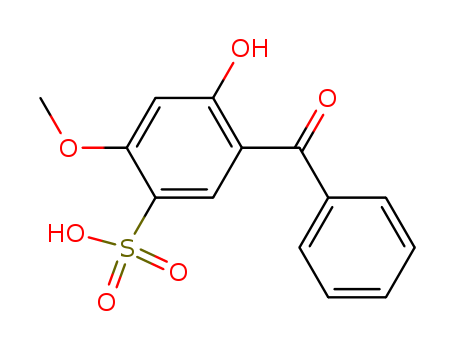
Best Quality Reputable Factory Supply Uv Absorber Bp-4 4065-45-6 with Cheap Price
- Molecular Formula:C14H12O6S
- Molecular Weight:308.312
- Appearance/Colour:white to yellowish powder
- Vapor Pressure:0Pa at 25℃
- Melting Point:170 °C
- Refractive Index:1.5300 (estimate)
- Boiling Point:0°C
- PKA:-0.70±0.50(Predicted)
- Flash Point:0°C
- PSA:109.28000
- Density:1.449 g/cm3
- LogP:2.95930
2-Hydroxy-4-methoxybenzophenone-5-sulfonic acid(Cas 4065-45-6) Usage
|
Manufacturing Process
|
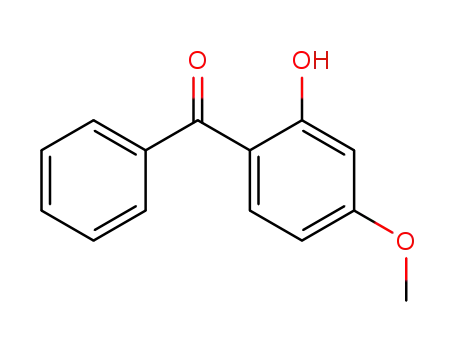
663 g of dichloroethane and 74.6 g 2-hydroxy-4-methoxybenzophenone were charged into a 3-neck flask equipped with stirrer, thermometer, reflux condenser and dropping funnel and a heating mantle. The solution was heated to the reflux temperature (85°C to 86°C) and was dehydrated by distilling off 66.5 g 1,2-dichloroethane. While maintaining at reflux, 30 g chlorosulfonic acid was added slowly over a period of about two hours. The rate of addition was regulated by the speed of evolution of the HCl. After all the chlorosulfonic acid was added, the charge was still maintained at reflux for an additional 15 minutes to remove traces of HCl. It was then cooled to 5°C and filtered. The filter cake was washed with 500 g cold 1,2-dichloroethane and dried. 98 g of product were obtained.
|
|
Flammability and Explosibility
|
Nonflammable
|
|
Contact allergens
|
BZP-4 is widely used in cosmetics, particularly shampoos and hair products. Cross-reactivity is rarely expected in patients photoallergic to ketoprofen.
|
|
Brand name
|
Sungard (Bayer).
|
|
General Description
|
Sulisobenzone is a broad spectrum organic UV filter in sunscreen formulations, known to absorb deleterious UV light.
|
InChI:InChI=1/C14H12O6S/c1-20-12-8-11(15)10(7-13(12)21(17,18)19)14(16)9-5-3-2-4-6-9/h2-8,15H,1H3,(H,17,18,19)/p-1
4065-45-6 Relevant articles
Preparation method of sun-screening agent 2-hydroxy-4-methoxy-5-sulfo benzophenone
-
Paragraph 0052; 0054-0055, (2021/02/06)
The invention provides a preparation met...
Method for synthesizing BP-4 using microreactor
-
Paragraph 0031-0036, (2020/01/25)
The invention discloses a method for syn...
Synthesis process for 2-hydroxy-4-methoxybenzophenone-5-sulfonic acid
-
Paragraph 0011, (2016/11/28)
The invention discloses a synthesis proc...
Cosmetic compositions
-
, (2014/07/08)
Suggested is a cosmetic compositions com...
4065-45-6 Upstream products
4065-45-6 Downstream products
-
19337-04-3
2--4-methoxy-5-sulfo-benzophenon
-
121715-18-2
5-benzoyl-4-hydroxy-2-methoxy-benzenesulfonyl chloride
-
817203-48-8
C14H11O6S(1-)*C18H12Cl3S(1+)
-
1243203-31-7
1-[2-hydroxy-4-methoxy-5-(3-nitrophenylazo)phenyl]-1-phenylmethanone
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego