Factory Sells Quality Manufacturer Supply Palmitol 36653-82-4 with Fast Delivery
- Molecular Formula:C16H34O
- Molecular Weight:242.445
- Appearance/Colour:white solid
- Vapor Pressure:<0.01 mm Hg ( 43 °C)
- Melting Point:49-51 °C
- Refractive Index:nD79 1.4283
- Boiling Point:310.9 °C at 760 mmHg
- PKA:15.20±0.10(Predicted)
- Flash Point:135 °C
- PSA:20.23000
- Density:0.818 g/cm3
- LogP:5.46000
- IDLH:114
- IDLH:57
- IDLH:2554
Palmitol(Cas 36653-82-4) Usage
|
Description
|
Palmitol is a C-16 fatty alcohol. At room temperature, cetyl alcohol takes the form of a waxy white solid or flakes. |
|
Flammability and Explosibility
|
Notclassified
|
|
Uses
|
Palmitol is used in the cosmetic industry as an opacifier in shampoos, or as an emollient, emulsifier or thickening agent in the manufacture of skin creams and lotions. |
|
Incompatibilities
|
Incompatible with strong oxidizing agents. Cetyl alcohol is responsible for lowering the melting point of ibuprofen, which results in sticking tendencies during the process of film coating ibuprofen crystals.
|
|
Regulatory Status
|
Included in the FDA Inactive Ingredients Database (ophthalmic preparations, oral capsules and tablets, otic and rectal preparations, topical aerosols, creams, emulsions, ointments and solutions, and vaginal preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Nonmedicinal Ingredients.
|
|
Who Evaluation
|
Evaluation year: 1997
|
InChI:InChI=1/C16H34O/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17/h17H,2-16H2,1H3
36653-82-4 Relevant articles
Effect of cetostearyl alcohol on stabilization of oil-in-water emulsion: I. Difference in the effect by mixing cetyl alcohol with stearyl alcohol
S Fukushima, M Takahashi, M Yamaguchi
Journal of Colloid and Interface Science Volume 57, Issue 2, November 1976, Pages 201-206
It is known that an oil-in-water emulsion increases in consistency and stability on addition of cetostearyl alcohol. When either cetyl alcohol or stearyl alcohol was added individually, the emulsion stability decreased. On storage at room temperature, unstable emulsions decreased in consistency and many particles (not visible immediately after preparation) appeared. The particles were determined to be crystals of the alcohol added. When both alcohols were included in the formulation simultaneously in the appropriate ratio, the emulsions were stable and did not show such changes. This difference in stability can be explained in relation to polymorphism of the alcohols.
Discovery of Anti-TNBC Agents Targeting PTP1B: Total Synthesis, Structure-Activity Relationship, in Vitro and in Vivo Investigations of Jamunones
Hu, Caijuan,Li, Guoxun,Mu, Yu,Wu, Wenxi,Cao, Bixuan,Wang, Zixuan,Yu, Hainan,Guan, Peipei,Han, Li,Li, Liya,Huang, Xueshi
supporting information, p. 6008 - 6020 (2021/05/06)
Twenty-three natural jamunone analogues ...
36653-82-4 Upstream products
-
64512-29-4
hexadeca-2,4,6,8,10,12,14-heptaenal
-
629-80-1
n-hexadecylaldehyde
-
64437-47-4
trans-hexadec-9-en-1-ol
-
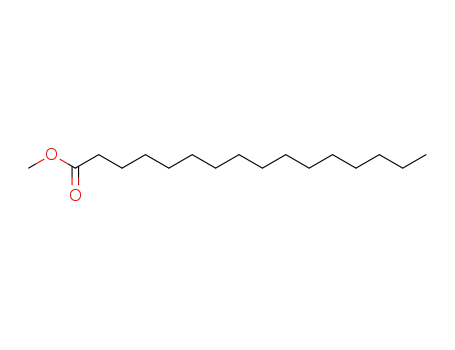
112-39-0
hexadecanoic acid methyl ester
36653-82-4 Downstream products
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego