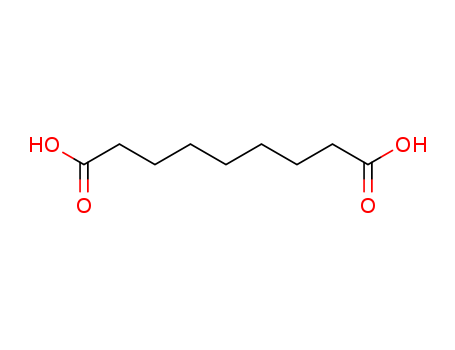
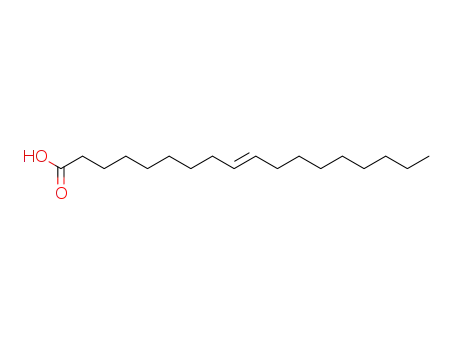High Purity Factory Supply High Purity Azelaic Acid 123-99-9 with Safe Delivery
- Molecular Formula:C9H16O4
- Molecular Weight:188.224
- Appearance/Colour:white to cream solid
- Vapor Pressure:<1 mm Hg ( 20 °C)
- Melting Point:98-103 ºC
- Refractive Index:1.4303
- Boiling Point:370.5 ºC at 760 mmHg
- PKA:4.53, 5.33(at 25℃)
- Flash Point:192.1 ºC
- PSA:74.60000
- Density:1.131 g/cm3
- LogP:1.88630
Azelaic acid(Cas 123-99-9) Usage
|
Indications
|
Azelaic acid (Azelex) is a naturally occurring dicarboxylic acid produced by the yeast Malassezia furfur. Azelaic acid inhibits tyrosinase, a rate-limiting enzyme in the synthesis of the pigment melanin. This may explain why diminution of melanin pigmentation occurs in the skin of some patients with pityriasis versicolor, a disease caused by M. furfur. Azelaic acid is bacteriostatic against a number of species thought to participate in the pathogenesis of acne, including Propionibacterium acnes. The drug may also reduce microcomedo formation by promoting normalization of epidermal keratinocytes.
|
|
Production Methods
|
Azelaic acid is industrially produced by the ozonolysis of oleic acid. The side product is nonanoic acid. It is produced naturally by Malassezia furfur (also known as Pityrosporum ovale), a yeast that lives on normal skin. The bacterial degradation of nonanoic acid gives azelaic acid.
|
|
Preparation
|
Azelaic acid is made by the ozonolysis of oleic acid:
|
|
Therapeutic Function
|
Antiacne, Depigmentor
|
|
Synthesis Reference(s)
|
Journal of the American Chemical Society, 77, p. 4846, 1955 DOI: 10.1021/ja01623a048Organic Syntheses, Coll. Vol. 2, p. 53, 1943
|
|
Biochem/physiol Actions
|
Azelaic acid is a potent inhibitor of 5α-reductase activity. It is a reversible competitive inhibitor of thioredoxin reductase in human melanoma cells.
|
|
Safety Profile
|
Low toxicity by ingestion. A skinand eye irritant. Closely related to glutaric acid and adipicacid. Combustible when exposed to heat or flame; canreact with oxidizing materials.
|
|
Application
|
Azelaic acid, also known as azalea acid, is a white to slightly yellow powder. Azelaic acid is a medium-long chain dibasic acid[1]. In recent years, with the rapid development of the organic synthetic chemical industry, the demand for medium and long chain dibasic acids is increasing. The medium and long chain dibasic acids and their derivatives have a wide range of industrial applications and a broad product market.
|
|
Brand name
|
Azelex (Allergan); Finacea (Intendis);Skinoren.
|
|
Biotechnological Applications
|
In plants, azelaic acid serves as a "distress flare" involved in defense responses after infection. It serves as a signal that induces the accumulation of salicylic acid, an important component of a plant's defensive response.
|
InChI:InChI=1/C9H16O4/c10-8(11)6-4-2-1-3-5-7-9(12)13/h1-7H2,(H,10,11)(H,12,13)/p-2
123-99-9 Relevant articles
A New and Efficient Approach to Macrocyclic Keto Lactones
Karim, Mohammad R.,Sampson, Paul
, p. 598 - 605 (1990)
A new and efficient method for macrolact...
γ-hydroxyalkenals are oxidatively cleaved through Michael addition of acylperoxy radicals and fragmentation of intermediate β-hydroxyperesters
Balamraju, Yuvaraju N.,Sun, Mingjiang,Salomon, Robert G.
, p. 11522 - 11528 (2004)
Oxidative cleavage of arachidonate (C20)...
Microbial synthesis of medium-chain α,ω-dicarboxylic acids and ω-aminocarboxylic acids from renewable long-chain fatty acids
Song, Ji-Won,Lee, Jung-Hoo,Bornscheuer, Uwe T.,Park, Jin-Byung
, p. 1782 - 1788 (2014)
Biotransformation of long-chain fatty ac...
Preparation, characterization, and theoretical studies of azelaic acid derived from oleic acid by use of a novel ozonolysis method
Kadhum, Abdul Amir H.,Wasmi, Bilal A.,Mohamad, Abu Bakar,Al-Amiery, Ahmed A.,Takriff, Mohd S.
, p. 659 - 668 (2012)
Environmentally friendly manufacture of ...
Simultaneous Enzyme/Whole-Cell Biotransformation of C18 Ricinoleic Acid into (R)-3-Hydroxynonanoic Acid, 9-Hydroxynonanoic Acid, and 1,9-Nonanedioic Acid
Cha, Hee-Jeong,Seo, Eun-Ji,Song, Ji-Won,Jo, Hye-Jin,Kumar, Akula Ravi,Park, Jin-Byung
, p. 696 - 703 (2018)
Regiospecific oxyfunctionalization of re...
Oxidative carbon-carbon bond cleavage of 1,2-diols to carboxylic acids/ketones by an inorganic-ligand supported iron catalyst
Chen, Weiming,Xie, Xin,Zhang, Jian,Qu, Jian,Luo, Can,Lai, Yaozhu,Jiang, Feng,Yu, Han,Wei, Yongge
, p. 9140 - 9146 (2021/11/23)
The carbon-carbon bond cleavage of 1,2-d...
123-99-9 Upstream products
-
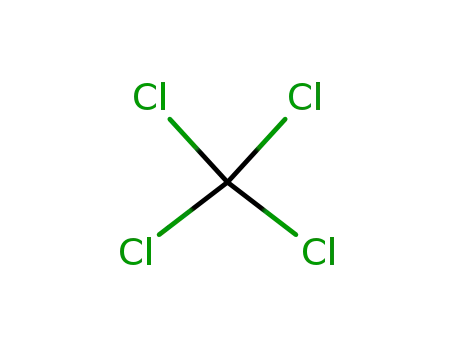
56-23-5
tetrachloromethane
-
112-79-8
Elaidic acid
-
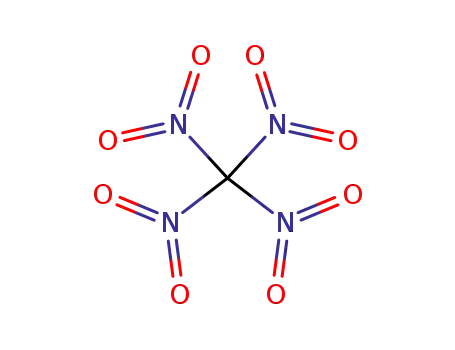
509-14-8
tetranitromethane
-
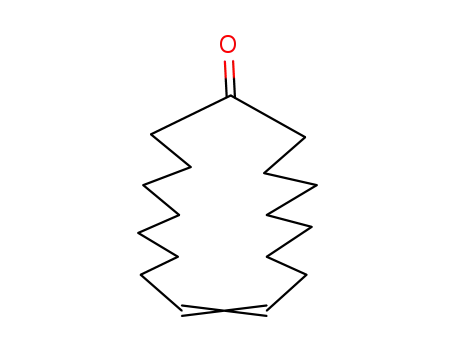
74244-64-7
cycloheptadec-9-en-1-one
123-99-9 Downstream products
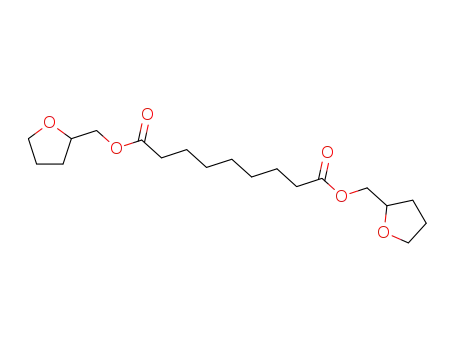
-
83327-23-5
Azelainsaeure-ditetrahydrofurfurylester
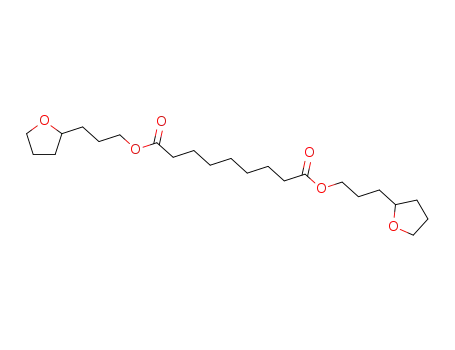
-
7507-11-1
azelaic acid bis-(3-tetrahydro[2]furyl-propyl ester)
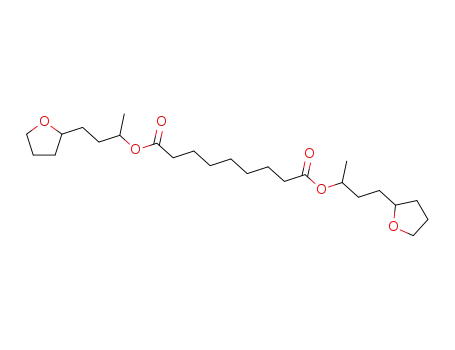
-
7702-52-5
azelaic acid bis-(1-methyl-3-tetrahydro[2]furyl-propyl ester)
-
2104-19-0
azelaic monomethyl ester
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego