|
Flavors and fragrances
|
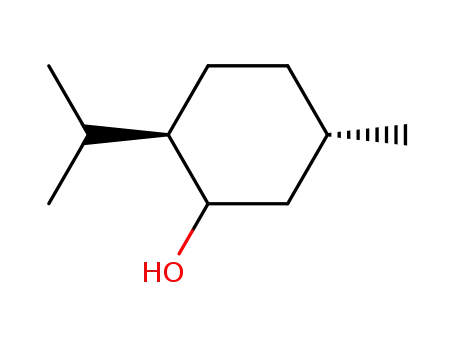
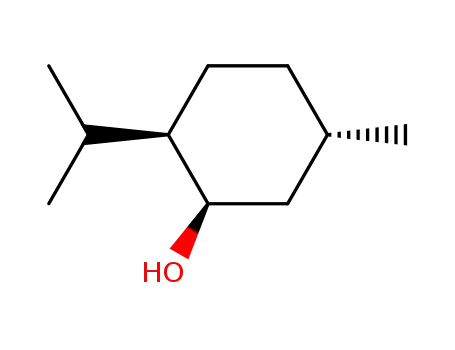
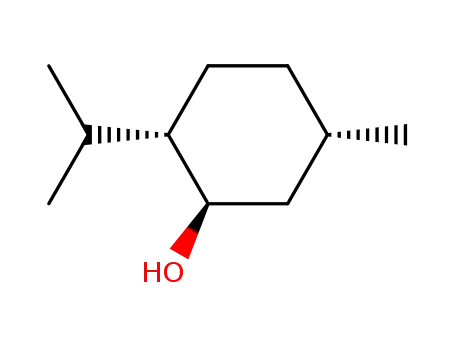
Menthol is a cyclic terpene alcohol flavors and fragrances with its appearance exhibiting colorless needle or shuttle columnar crystal or white crystalline powder. It has special aroma of mint with its taste being hot at first but then being cool later. It exhibit neutralizing reaction in the ethanol solution, being extremely easily soluble in ethanol, chloroform, petroleum ether, ether, liquid paraffin or volatile oil and being very slightly soluble in water.
Its molecular structure contains three asymmetric carbon atoms, so it has four stereoisomers with their aroma intensity and type and cool feeling being different. There are two common types: natural menthol and synthetic menthol. Natural menthol is a kind of saturated ring alcohol obtained through the freezing & crystallization, separation and refinement of peppermint oil with the optical rotation being L; synthetic menthol is made from taking lemon eucalyptus oil as raw material.
Its main component can be manufactured from the ring closing reaction of citronellal to obtain isopulegol, followed by hydrogenation, or through the synthesis of thymol from m-cresol with catalytic hydrogenation resulting in four menthol mixture of isomers with the precise vacuum distillation obtaining defined synthetic menthol, being racemate with no optical rotation. It has bactericidal and anti-corrosion effect and can be used as the fragrance of toothpaste, perfume, beverages and confectionery. In the medicine, it can be used for making cool oil, painkillers, mouthwash, and so on.
The main applications are as follows:
1. Menthol can act on the skin sensory nerve end, making the skin surface to produce cool feeling, achieving the purpose of itching prevention. Topical administration to the affected area can be used for the treatment of urticaria, prurigo, prickly heat, rash, neurodermatitis and skin pruritus and so on.
2. It can be used for the treatment of headache, nasal congestion and sore throat. This product has pleasant aroma and has curing effect on treating cold and wind heat. It has effects of refreshing the mind and clearing the throat. |
|
First aid of poisoning
|
Topical application of menthol have effects of promoting blood circulation and anti-inflammatory, anti-itching and can be applied to anti-inflammatory, antipruritic, analgesic and reducing swelling and so on.
【Diagnosis】
1. Oral administration of large amount of menthol or inhaling of large amount of menthol gas can lead to gastrointestinal symptoms such as nausea, vomiting and abdominal pain as well as nervous system symptoms such as excitement, tremor, dizziness, vertigo, hand and foot numbness, swing gait, lethargy, breathing changes, facial flushing, reduced blood pressure, muscle rigidity or relaxation, occasional being not able to speak; children, after taking a large dose, can have coma. But it can be generally restored within a few hours.
2. Patients allergic to this chemical can get with eczema with unbearable itching feeling.
3. Small infants, sometimes due to nasal mint liquid or ointment, can get glottis spasm, accompanied with bruising and suffocation as well as secretion of large amounts of mucus. In severe cases, it may be occurred of respiratory and cardiac arrest.
【Treatment principles】
1. Gastric lavage; conduct catharsis with salt cathartic. Avoid using fats and oils.
2. Apply intravenous rehydration to promote excretion.
3. In cases of allergic symptoms, we should treat with antihistamines such as diphenhydramine, chlorpheniramine, etc.; if necessary, we can apply hydrocortisone or dexamethasone intravenously. |
|
Detection method
|
(1) Chemical qualitative method
① take 1 g of this product, add 20 mL of sulfuric acid to dissolve it, so the reaction mixture becomes orange-red. After 24h, there will be colorless oil layer of non-menthol aroma precipitated out (the distinction from thymol).
② take 50 mg of this product, add 1mL of ice acetic acid to dissolve it; Addition of 6 drops of sulfuric acid and 1 drop of nitric acid to the cold mixture will lead to only slightly yellow color (the difference from thymol).
(2) thin-layer chromatography: take appropriate amount of menthol and dissolve in anhydrous ethanol, make a solution containing 6 mg menthol per mL for spotting. TLC: silica gel G manual plating, make it dry; have it subjected to activation in 105 °C for 1 hour and standby. Developing agent A: n-hexane-ethyl acetate-benzene (43: 4.5: 4); Developing agent B: petroleum-ether-ethyl acetate-chloroform (25: 2.5: 5.5). Coloration reagent: 5% vanillin ethanol solution-concentrated sulfuric acid (100: 6). Coloration: heating at 105 ℃ for 6~10min, exhibiting a circular blue spots.
(3) Determination of physical constants: melting point: 42 ℃~44 °C. Specific rotation: take this product for accurately weighing; add ethanol to make a solution of 0.1 g/mL. Measure according to the method, the specific rotation is-49 ° ~-50 °.
(4) non-volatile matter: take 2 g of this product and place it in the pre-weighed evaporating dish; have it heated in the water bath so it is slowly evaporated; dried at 105 ℃ to constant weight with the leftover residue not exceeding 1mg. |
|
History
|
In the world of aromatic chemicals, menthol is often described as a unique source
of skin and mucous membranes. Menthol is mainly extracted from natural plants.
The vast majority of natural menthol in the global market is extracted from numerous Asian mint. In 2006, the world’s natural menthol production is 12,800?tons or
more. However, due to various factors, the production of natural menthol is becoming less and less. Since the 1960s, Japan, Germany, and other countries had developed the synthetic menthol products. In 1974 the German company Symrise and
Japan Takasago Fluidic Systems company launched chemical synthesis of menthol.
At present, attention has been paid to the study of menthol analogy substances and
the effect of the changes of molecular structure on the aroma and cool sensation.
Some studies have shown that hydroxyl groups located in the branched or 1, 4 chain
cannot show a sense of cool. The researchers believed that menthol analogy substances with optical activity should be able to show different, surprising cooling
effect, and the structure of hydroxyl alkyl groups is the key point to have a cooling
effect. The Japanese chemist Ryoji Noyo and his colleagues found that in
BINAP and rhodium complexes as catalysts for asymmetric hydrogenation reaction, the synthesis of menthol is very effective. Because of the contribution of asymmetric organic synthesis, they won the 2001 Nobel Prize in Chemistry. |
|
Preparation
|
By hydrogenation of thymol. |
|
Indications
|
For external application, it is applied locally and often used to relieve pain and itching. A nasal drip is used in the head cold. Inhalation or spray is used for sore throat.
Oral administration can promote digestion. |
|
Air & Water Reactions
|
Insoluble in water. |
|
Reactivity Profile
|
DL-Menthol is incompatible with butyl chloral hydrate, camphor, phenol, chloral hydrate, Exalgine, betanaphthol, resorcinol or thymol in triturations; potassium permanganate, chromium trioxide and pyrogallol. DL-Menthol is also incompatible with strong oxidizers. |
|
Hazard
|
Irritant to mucous membranes on inhalation. |
|
Fire Hazard
|
DL-Menthol is combustible. |
|
Flammability and Explosibility
|
Notclassified |
|
Biochem/physiol Actions
|
Menthol exhibits antifungal action on the membrane permeability and the cell wall consistency, this might leads to cell death or inhibition of the C. albicans filamentation. Therefore, it can be used as a potential therapeutic agent for candidiasis. In addition to antifungal activity, menthol also exhibits anesthetic, antispasmodic, anti-ulcer and antiviral properties. It is considered as a safe compound for animal life. Therefore, Menthol is widely used in pharmaceuticals, cosmetics and food industries. |
|
Pharmacology
|
Menthol has a wide range of pharmacological effects. Menthol can stimulate the
nerve endings of skin and slowly penetrate into the skin, resulting in prolonged
congestion and reflex caused by deep vascular changes, adjusting the vascular function, and achieving therapeutic effects in topical application. Topical application of
compound has anti-inflammatory, analgesic, and anesthetic effects.In respiratory system: For treatment of bronchitis, it could reduce the respiratory
tract foam sputum and increase the effective ventilation cavity road. For treatment
of rhinitis and laryngitis, it exerts mitigation by promoting secretion and diluting
viscous mucus.In digestive system: Menthol showed the powerful effect on bile.In central nervous system: A small amount of peppermint can stimulate the central nervous system through the peripheral nerve to expand the skin capillaries, promote the secretion of sweat glands, increase heat dissipation, and show sweating
antipyretic effect.In addition, menthol has penetration-enhancing effect on many kinds of drugs;
the mechanism is related to the changes of the ultrastructure of the skin, which is
expected to be widely used in transdermal drug delivery agents. In vitro experiments, it had strong antibacterial activity against Staphylococcus aureus and
Bacillus proteus. Recently, British scientists found that mint leaves can prevent cancer lesions in the blood vessel growth to decrease blood supply for, showing a certain anti-tumor effect |
|
Safety Profile
|
Poison by intravenous
route. Moderately toxic by ingestion and
intraperitoneal routes. A severe eye irritant.
Incompatible with phenol, p-naphthol,
resorcinol or thymol in trituration,
potassium permanganate, chromium
trioxide, pyrogallol. Combustible liquid.
\When heated to decomposition it emits
acrid smoke and irritating fumes. |
|
Chemical properties
|
L-form appears as colorless needle crystal. The melting point is 42-43 °C; the boiling point is 216 °C, the specific rotation:-51 °-45 °; the flash point: 93 °C; the relative density: 0.8810; it is soluble in ethanol and is miscible with oil. It has cool, fresh, pleasant mint characteristic aroma with sweet spiky gas. It gives people a cold feeling with aroma penetrating through the hair, but not lasting. The taste is also fresh sweet cool taste. |
|
Physical properties
|
Appearance: colorless or white acicular prismatic crystals or white crystalline powder. Solubility: easily dissolved in ethanol, chloroform, ether, liquid paraffin, or
volatile oil and soluble in water. Odor: a cool, refreshing, and pleasant mint aroma,
sweet odor, and taste cool early after burning. Boiling point: 212?°C. Melting point:
41–43?°C. Specific optical rotation: ?49 to ?50°. |
|
Aroma threshold values
|
Detection: 950 ppb to 2.5 ppm; aroma characteristics at 1.0%: cooling menthol with a penetrating minty
eucalyptus note. |
|
Taste threshold values
|
Taste characteristics at 25 ppm: cooling, camphoreous, minty with a clean eucalyptus note. |
|
General Description
|
White crystalline solid with a peppermint odor and taste. |
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego