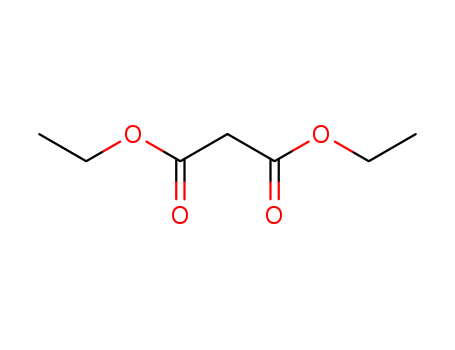
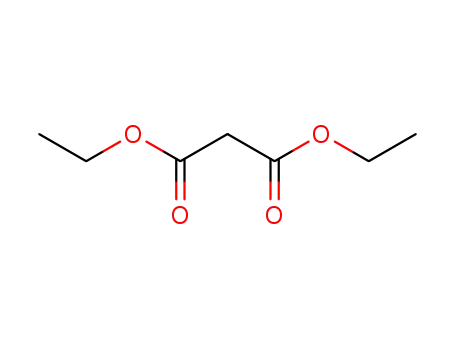
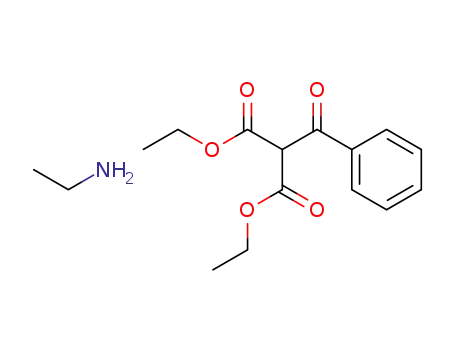
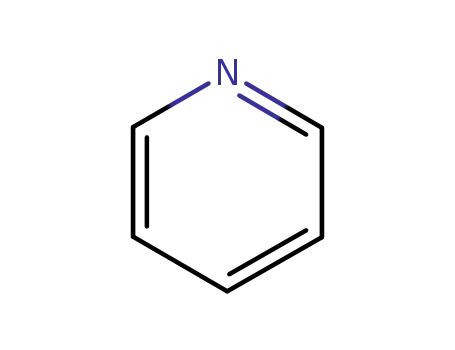
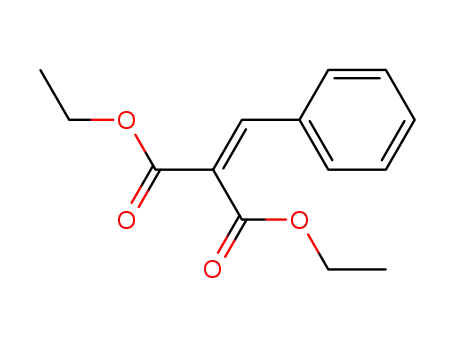
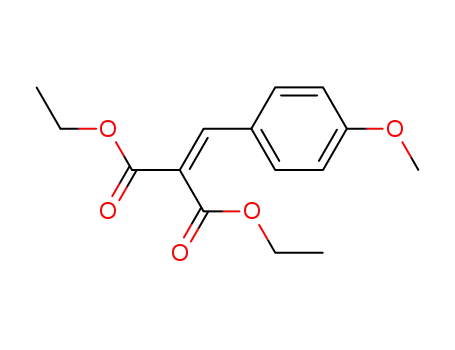
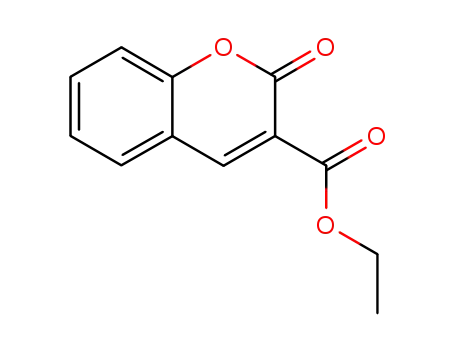
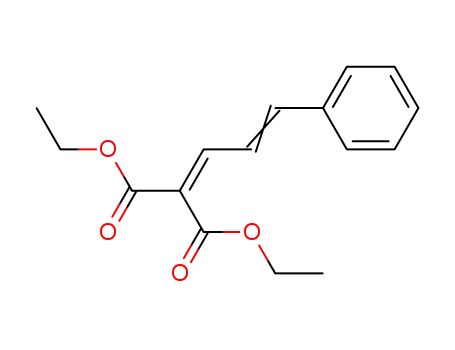
A General and Mild Copper-Catalyzed Arylation of Diethyl Malonate
Edward J. HennessyStephen L. Buchwald
, Org. Lett. 2002, 4, 2, 269–272
A general method for the synthesis of α-aryl malonates is described. The coupling of an aryl iodide and diethyl malonate in the presence of Cs2CO3 and catalytic amounts of copper(I) iodide and 2-phenylphenol affords the α-aryl malonate in good to excellent yields. The mild reaction conditions and high levels of functional group compatibility make this an attractive synthetic alternative to previous methods.
The dehydrodimerisation of diethyl malonate in six different designs of cell
R.E.W. Jansson , N.R. Tomov 1
, Electrochimica Acta Volume 25, Issue 5 , May 1980, Pages 497-503
The dehydrodimerisation of diethyl malonate to ethyl-1,1,2,2, ethane tetracarboxylate has been studied in six varieties of cell — a pump cell, a capillary gap cell, a bipolar trickle tower, a paralled plate cell, a parallel plate cell with turbulence promoters and a poorly stirred tank — with potassium iodide as electrolyte. At low conversion the simplest cells give the most ideal performance, since the anolyte and catholyte layers are naturally segregated from the bulk and the stable malonate anion reacts with electrogenerated iodine in the reactor loop where mixing is good.
Synthesis of coumarin-3-carboxylic esters via FeCl3-catalyzed multicomponent reaction of salicylaldehydes, Meldrum's acid and alcohols
He, Xinwei,Shang, Yongjia,Zhou, Yao,Yu, Zhiyu,Han, Guang,Jin, Wenjing,Chen, Jiaojiao
, p. 863 - 868 (2015)
A FeCl3-catalyzed multicomponent reactio...
A Mild, Simple, and Convenient Synthesis of Diesters from Malonyl or Succinyl Dichloride and Alcohols Catalyzed by Potassium Tetracarbonylhydridoferrate, KHFe(CO)4, as a New Catalyst
Shim, Sang Chul,Huh, Keun Tae,Park, Woo Hyun
, p. 59 (1987)
A large variety of alcohols react with a...
Promoting charge separation in donor-acceptor conjugated microporous polymers: Via cyanation for the photocatalytic reductive dehalogenation of chlorides
Deng, Jiyong,Fang, Zhengjun,Lan, Donghui,Liao, Yunfeng,Liu, Qingquan,Zhang, Weijie,Zhou, Xiang
, p. 7151 - 7159 (2021/11/17)
Conjugated microporous polymers (CMPs) h...
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego