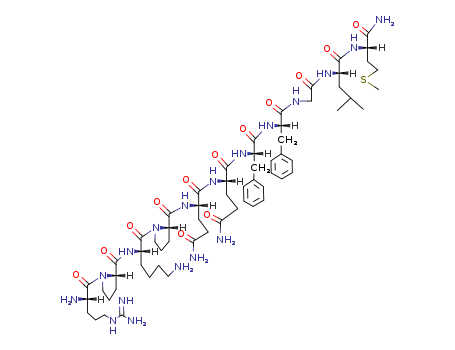
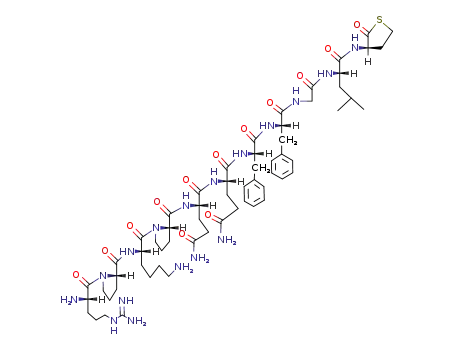
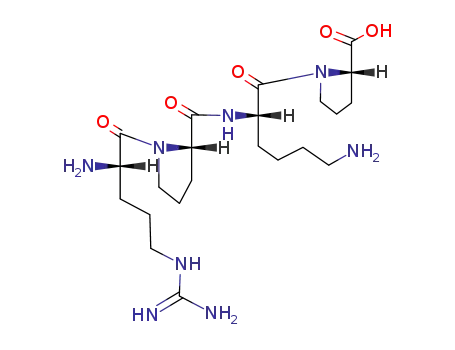
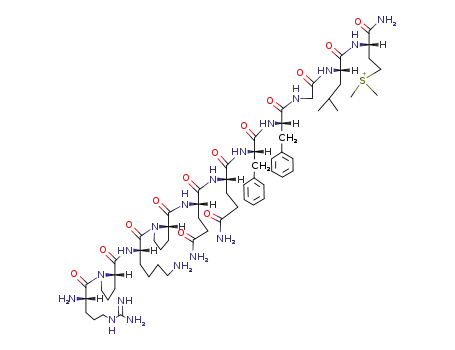
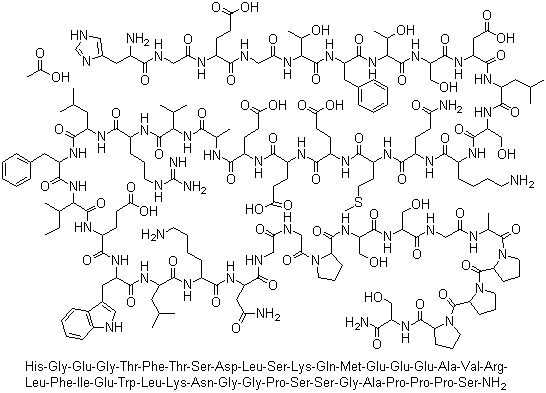
Chinese Manufacturer Supply SUBSTANCE P 33507-63-0 On Stock with Competitive Price
- Molecular Formula:C63H98N18O13S
- Molecular Weight:1347.65
- Appearance/Colour:white powder
- Melting Point:148 °C
- PKA:13.26±0.46(Predicted)
- PSA:544.43000
- Density:1.42 g/cm3
- LogP:4.19410
Substance P(Cas 33507-63-0) Usage
|
General Description
|
Substance P is a polypeptide consisting of 11 amino acidresidues. It has been implicated in the transmission of“painful” sensory information through the spinal cord tohigher centers in the central nervous system. Substance Pis localized in the primary afferent sensory fibers. Other pharmacologicaleffects are vasodilatation, stimulation of smoothmuscles, stimulation of salivary secretion, and diuresis. In addition,this neuropeptide contributes to some inflammatory responses.Approximately 50% of the known neuropeptides aresynthesized as biologically inactive glycine extended precursorsthat require a carboxy-terminal posttranslational amidationfor biological activity. Amidation enzymes are responsiblefor the conversion of the carboxyl group of the neuropeptide tothe corresponding amide group and include the two amidatingenzymes peptidylglycine α-monooxygenase (PAM) and peptidylamidoglycolatelyase (PGL), which work sequentially toproduce the inflammatory neuropeptide Substance P from aninactive precursor peptide. Much research is being performedto exploit this mechanism of inflammation. |
InChI:InChI=1/C63H98N18O13S/c1-37(2)33-45(57(89)74-41(53(68)85)27-32-95-3)73-52(84)36-72-54(86)46(34-38-15-6-4-7-16-38)78-58(90)47(35-39-17-8-5-9-18-39)79-56(88)42(23-25-50(66)82)75-55(87)43(24-26-51(67)83)76-59(91)49-22-14-31-81(49)62(94)44(20-10-11-28-64)77-60(92)48-21-13-30-80(48)61(93)40(65)19-12-29-71-63(69)70/h4-9,15-18,37,40-49H,10-14,19-36,64-65H2,1-3H3,(H2,66,82)(H2,67,83)(H2,68,85)(H,72,86)(H,73,84)(H,74,89)(H,75,87)(H,76,91)(H,77,92)(H,78,90)(H,79,88)(H4,69,70,71)/t40-,41-,42-,43-,44-,45-,46-,47-,48-,49-/m0/s1
33507-63-0 Relevant articles
Tachykinin NK-1 receptor probed with constrained analogues of substance P
Sagan, Sandrine,Josien, Hubert,Karoyan, Philippe,Brunissen, Alie,Chassaing, Gerard,Lavielle, Solange
, p. 2167 - 2178 (2007/10/03)
The action of rotameric probes introduce...
Solid Phase Synthesis of Substance P and Its Analogues Employing 9-Fluorenylmethoxycarbonylamino Acid Active Esters
Sivanandaiah, K. M.,Rangaraju, N. S.
, p. 1045 - 1049 (2007/10/02)
Substance P and six of its analogues con...
Catalytic Transfer Hydrogenation in Synthesis of Substance P
Sivanandaiah, K. M.,Rangaraju, N. S.
, p. 787 - 792 (2007/10/02)
The utility of catalytic transfer hydrog...
Homocysteine thiolactone as precursor of methionine amide: Application to the modification of peptides of the tachykinin family
Chassaing,Lavielle,Julien,Marquet
, p. 623 - 626 (2007/10/02)
-
33507-63-0 Process route
Conditions
| Conditions |
Yield |
|
With
water;
at 40 ℃;
for 2h;
|
|
-
-
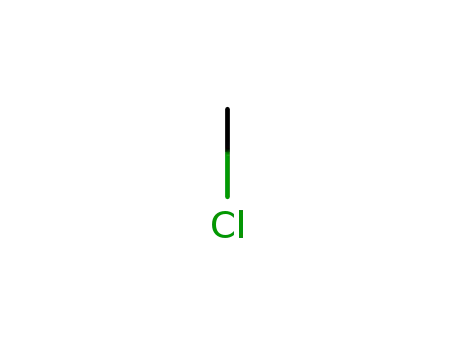
74-87-3
methylene chloride
Conditions
| Conditions |
Yield |
|
In
ammonium hydroxide;
|
|
33507-63-0 Upstream products
33507-63-0 Downstream products
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego