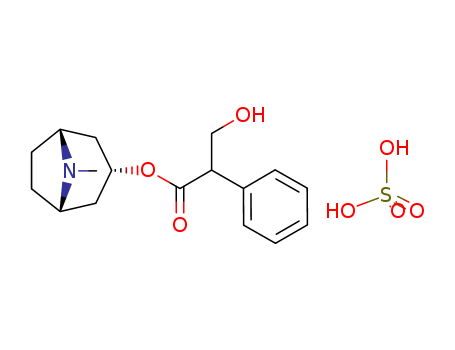
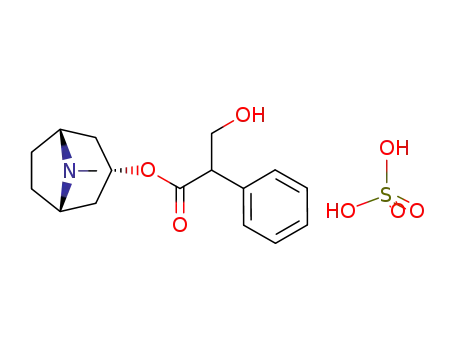
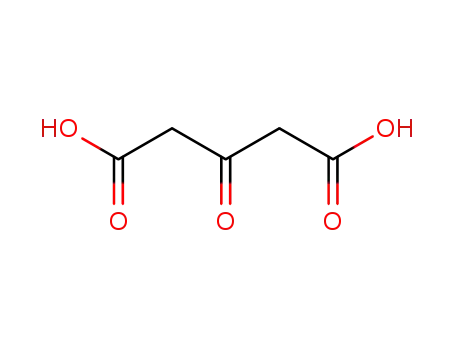
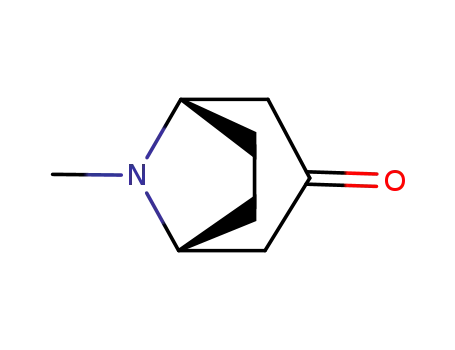
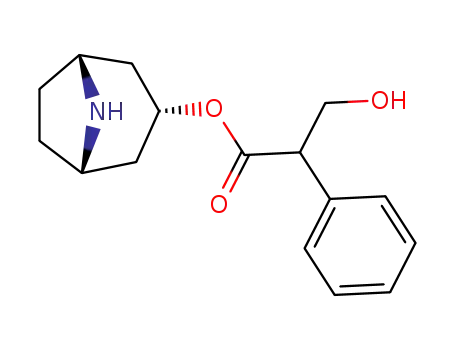
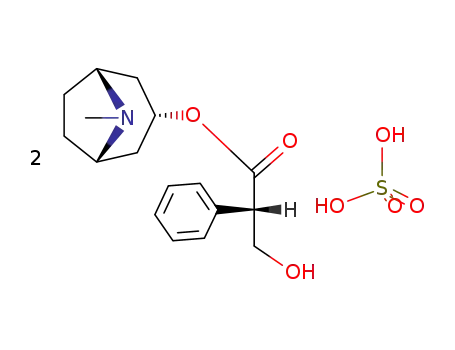
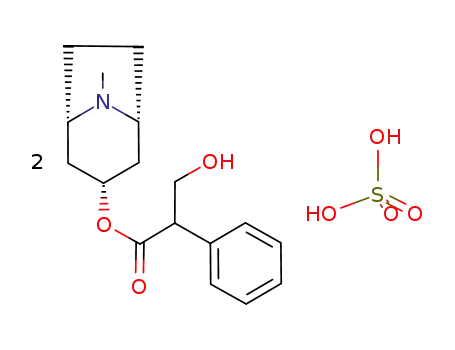
Factory supply Atropine sulfate 55-48-1 with sufficient production capacity
- Molecular Formula:C17H23NO3*H2O4S
- Molecular Weight:387.454
- Appearance/Colour:White crystalline powder
- Vapor Pressure:9.1E-28mmHg at 25°C
- Melting Point:189-192 °C (A)(lit.)
- Refractive Index:1.6900 (estimate)
- Boiling Point:806.7 °C at 760 mmHg
- Flash Point:441.7 °C
- PSA:182.52000
- Density:1.1172 (rough estimate)
- LogP:4.16560
Atropine sulfate(Cas 55-48-1) Usage
|
General Description
|
Atropine sulfate (Atropisol)is prepared by neutralizing atropine in acetone or ethersolution with an alcoholic solution of sulfuric acid, withcare used to prevent hydrolysis. The salt occurs as colorlesscrystals or as a white, crystalline powder. It is efflorescentin dry air and should be protected from light to preventdecomposition.Atropine sulfate is freely soluble in water (1:0.5), in alcohol(1:5, 1:2.5 at boiling point), and in glycerin (1:2.5).Aqueous solutions are not very stable, although solutionsmay be sterilized at 120°C (15 lb pressure) in an autoclave if the pH is kept below 6. Sterilization probably is besteffected by the use of aseptic techniques and a bacteriologicalfilter. It has been suggested that no more than a 30-daysupply of an aqueous solution should be made and that forsmall quantities the best procedure is to use hypodermictablets and sterile distilled water. Kondritzer andZvirblis have studied the kinetics of alkaline and protoncatalyzedhydrolyses of atropine in aqueous solution. Theregion of maximal stability lies between pH 3 and approximately5. They have also proposed an equation to predictthe half-life of atropine undergoing hydrolysis at constantpH and temperature. |
|
Safety Profile
|
Poison by
subcutaneous, intravenous, and
intraperitoneal routes. Moderately toxic by
ingestion. Human (child) pulmonary system
effects by ingestion. Human systemic
effects: decreased body temperature, cardiac
arrhythmias. An experimental teratogen.
Other experimental reproductive effects. See
also ATROPINE. When heated to
decomposition it emits very toxic fumes of
NO,xand SOx. |
|
Veterinary Drugs and Treatments
|
The principal veterinary indications for systemic atropine include:
!!Preanesthetic to prevent or reduce secretions of the respiratory
tract
!!Treat sinus bradycardia, sinoatrial arrest, and incomplete AV
block
!!Differentiate vagally-mediated bradycardia for other causes
!!As an antidote for overdoses of cholinergic agents (e.g., physostigmine,
etc.)
!!As an antidote for organophosphate, carbamate, muscarinic
mushroom, blue-green algae intoxication
!!Hypersialism
!!Treatment of bronchoconstrictive disease |
InChI:InChI=1/2C17H23NO3.H2O4S/c2*1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12;1-5(2,3)4/h2*2-6,13-16,19H,7-11H2,1H3;(H2,1,2,3,4)/t2*13-,14+,15+,16?;
55-48-1 Relevant articles
Enantiomeric separation and simulation study of eight anticholinergic drugs on an immobilized polysaccharide-based chiral stationary phase by HPLC
Li, Meng,Zhang, Bo,Yu, Jia,Wang, Jian,Guo, Xingjie
, p. 11724 - 11731 (2018/07/25)
The enantiomeric separation of eight ant...
An anti-choline medicine preparation method of atropine sulfate
-
Paragraph 0039; 0040, (2017/03/08)
The invention provides a synchronizing m...
Preparation method for atropine sulfate
-
Paragraph 0011; 0014; 0017; 0023, (2017/06/02)
The invention discloses a preparation me...
CRYSTALLINE ATROPINE SULFATE
-
, (2014/07/21)
The present invention relates to crystal...
55-48-1 Process route
-
-
5908-99-6,55-48-1,300-40-3,620-61-1,1867-24-9,2472-17-5,2623-69-0,14844-27-0
atropine sulfate
Conditions
| Conditions |
Yield |
|
With
sulfuric acid;
In
dichloromethane; water; acetone;
at 29 - 42 ℃;
for 3.5h;
|
85 g
|
-
-
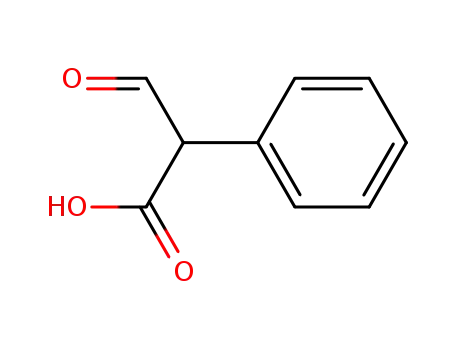
59216-85-2
α-formyl phenyl acetic acid
-
-
5908-99-6,55-48-1,300-40-3,620-61-1,1867-24-9,2472-17-5,2623-69-0,14844-27-0
atropine sulfate
Conditions
| Conditions |
Yield |
|
Multi-step reaction with 3 steps
1: sodium methylate / toluene / 5 h / 109 - 115 °C
2: sodium tetrahydroborate; methanol / dichloromethane / 4 h / 0 - 20 °C
3: sulfuric acid / water; dichloromethane; acetone / 3.5 h / 29 - 42 °C
With
methanol; sodium tetrahydroborate; sulfuric acid; sodium methylate;
In
dichloromethane; water; acetone; toluene;
|
|
55-48-1 Upstream products
55-48-1 Downstream products
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego